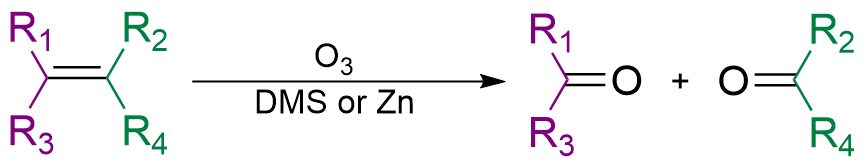|
2.2.2-Propellane
.2.2ropellane, formally tricyclo .2.2.01,4ctane is an organic compound, a member of the propellane family. It is a hydrocarbon with formula C8H12, or C2(C2H4)3. Its molecule has three rings with four carbon atoms each, sharing one C–C bond. This compound is unstable (although not as much as .1.1ropellane; however it is less persistent than .1.1ropellane). The bond angles on the shared carbons are considerably strained: three of them are close to 90°, the other three to 120°. The strain energy is estimated to be 93 kcal/mol (390 kJ/mol). Synthesis .2.2ropellane was first synthesized in 1973 by the group of Philip Eaton (who had earlier obtained cubane), according to the following scheme: : The synthesis begins with photochemical +2 ycloaddition of ethene on the cyclohexene derivative 1 to produce the bicyclic compound 2, followed by elimination reaction with potassium ''t''-butoxide of acetic acid to cyclobutene 3, followed by another cycloaddition with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propellane
In organic chemistry, propellane is any member of a class of polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon covalent bond. The concept was introduced in 1966 by D. Ginsburg Propellanes with small cycles are highly strained and unstable, and are easily turned into polymers with interesting structures, such as staffanes. Partly for these reasons, they have been the object of much research. Nomenclature The name derives from a supposed resemblance of the molecule to a propeller: namely, the rings would be the propeller's blades, and the shared C–C bond would be its axis. The bond shared by the three cycles is usually called the "bridge"; the shared carbon atoms are then the "bridgeheads". The IUPAC nomenclature of the homologue series of all-carbon propellanes would be called tricyclo .y.z.01,(x+2)lkane. More common in literature is the notation means the member of the family whose rings have ''x'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Livin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricyclic Compounds
Tricyclics are chemical compounds that contain three interconnected rings of atoms. Many compounds have a tricyclic structure, but in pharmacology, the term has traditionally been reserved to describe heterocyclic drugs. Among these are antidepressants, antipsychotics, anticonvulsants, and antihistamines (as antiallergens, anti- motion sickness drugs, antipruritics, and hypnotics/sedatives) of the dibenzazepine, dibenzocycloheptene, dibenzothiazepine, dibenzothiepin, phenothiazine, and thioxanthene chemical classes, and others. History * Promethazine and other first generation antihistamines with a tricyclic structure were discovered in the 1940s. * Chlorpromazine, derived from promethazine originally as a sedative, was found to have neuroleptic properties in the early 1950s, and was the first typical antipsychotic. * Imipramine, originally investigated as an antipsychotic, was discovered in the early 1950s, and was the first tricyclic antidepressant. * Carbamazepine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential (or, rarely, non-exponential) decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life (in exponential growth) is doubling time. The original term, ''half-life period'', dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to ''half-life'' in the early 1950s. Rutherford applied the principle of a radioactive element's half-life in studies of age determination of rocks by measuring the decay period of radium to lead-206. Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alicyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cycloheptane, cyclooctane, and so on. Bicyclic alkanes include bicycloundecane, decalin, and housane. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules. Otto Wallach, a German chemist, received the 1910 Nobel Prize in Chemistry for his work on alicyclic compounds. Cycloalkenes Monocyclic cycloalkenes are cyclopropene, cyclobutene, cyclopentene, cyclohexene, cycloheptene, cyclooctene, and so on. Bicyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are highly electrophilic at the carbon atom bonded with the heteroat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |