|
2,2'-Biphenylene Phosphorochloridite
2,2′-Biphenylene phosphorochloridite is the name for a polycyclic organophosphorus compound with the formula C12H8O2PCl. It is a precursor to di phosphite ligands such as BiPhePhos by reaction with suitable diols. 2,2′-Biphenylene phosphorochloridites, which is a white solid, is prepared from 2,2′- biphenol and phosphorus trichloride. It is prepared by the reaction of 2,2′-biphenol and phosphorus trichloride Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a ....{{cite journal, doi=10.1021/OM950549K, title=Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization, journal=Organometallics, volume=15, pages=835–847, year=1996, last1=Van Rooy, first1=Annemiek, last2=Kamer, first2=Paul C. J., last3=Van Leeuwen, first3=Piet W. N. M., last4=Goubitz, first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphite
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
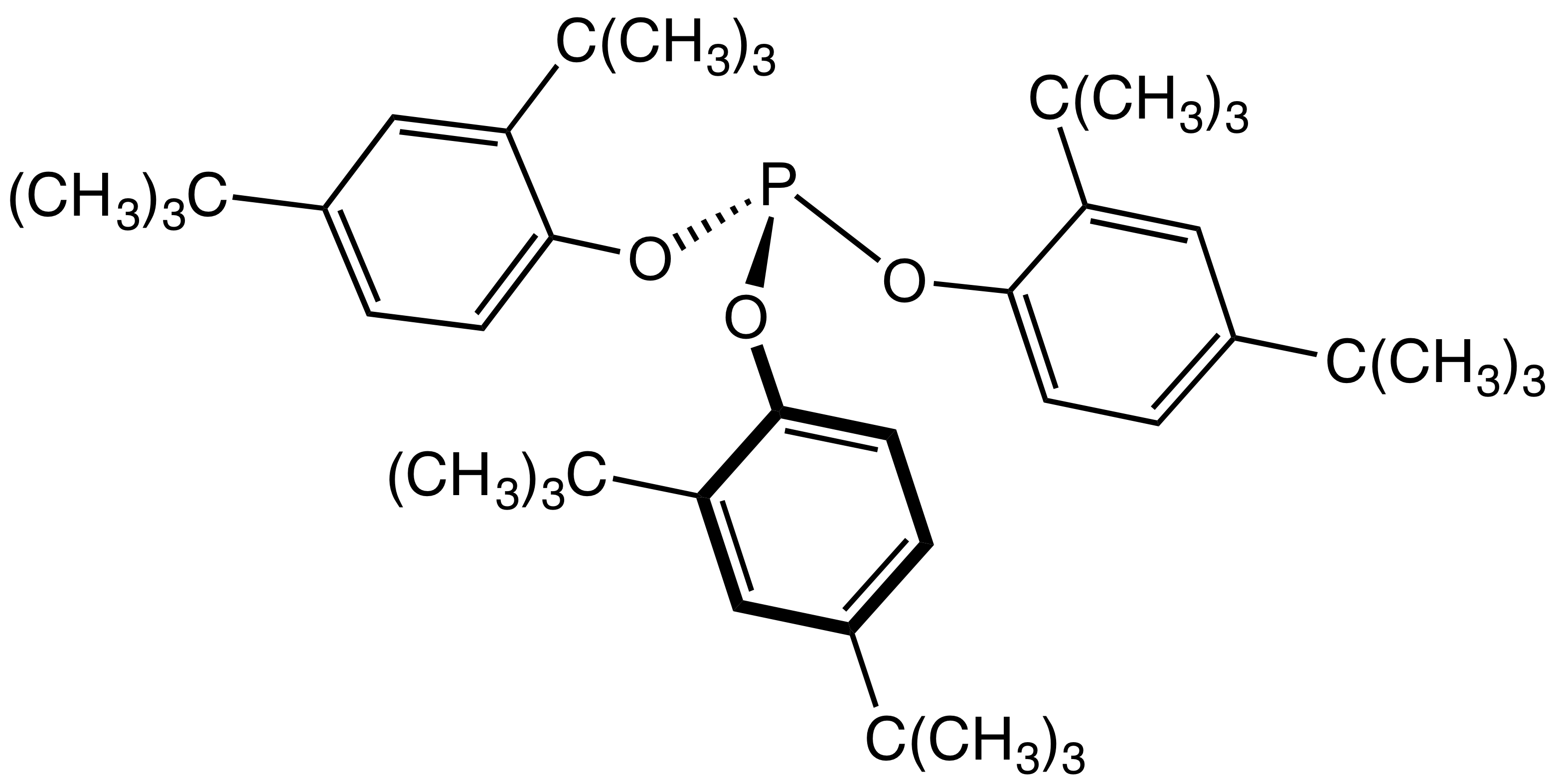
BiPhePhos
BiPhePhos is an organophosphorus compound that is used as a ligand in homogeneous catalysis. Classified as a diphosphite, BiPhePhos is derived from three 2,2'-biphenol groups, which constrain its shape in such a way to confer high selectivity to derived catalysts. Originally described by workers at Union Carbide, it has become a standard ligand in hydroformylation.{{cite journal, doi=10.1021/OM950549K, title=Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization, journal=Organometallics, volume=15, issue=2, pages=835–847, year=1996, last1=Van Rooy, first1=Annemiek, last2=Kamer, first2=Paul C. J., last3=Van Leeuwen, first3=Piet W. N. M., last4=Goubitz, first4=Kees, last5=Fraanje, first5=Jan, last6=Veldman, first6=Nora, last7=Spek, first7=Anthony L., url=http://dare.uva.nl/personal/pure/en/publications/bulky-diphosphite-modified-rhodium-catalyst-hydroformylation-and-characterization(c06c2654-cecb-4e97-84ba-f1fdfb51ad35).html See also *2,2'-Biphenylene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenol
In organic chemistry, a biphenol refers to compounds with the formula (C6H4OH)2. Such compounds formally result from the coupling of two phenols. {{short description, Chemical compound Three symmetrical isomers of biphenol exist: *2,2'-Biphenol ( RN 1806-29-7) m.p. 109 °C * 3,3'-Biphenol (RN 612-76-0) m.p. 124.8 °C *4,4'-Biphenol 4,4′-Biphenol is an organic compound which is a phenolic derivative of biphenyl. It is a colorless solid. 4,4′-Biphenol is prepared by dealkylation of the tetra-''t''-butyl derivative, generated by the oxidative coupling of 2,6-di-''tert''-b ... (RN 92-88-6) m.p. 283 °C Additionally, three unsymmetrical isomers of biphenol exist: * 2,3'-Biphenol (RN 31835-45-7) * 2,4'-Biphenol (RN 611-62-1) m.p. 162-163 °C * 3,4'-Biphenol (RN 18855-13-5) m.p. 190 °C Phenols Biphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2'-Biphenol
2,2′-Biphenol is an organic compound with the formula (C6H4OH)2. It is one of three symmetrical isomers of biphenol. A white solid, it is a precursor to di phosphite ligands that are used to support industrial hydroformylation catalysis. 244px, left, BiPhePhos is representative diphosphite ligand derived from 2,2′-biphenol. Synthesis Ring-opening of Dibenzofuran Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of th ... affords 2,2′-biphenol. Alternatively, it can be produced from 2,4-di-''tert''-butylphenol in two steps. The first step entails oxidative coupling to give the 2,2′-biphenol with four ''tert''-Bu substituents. This species then undergoes debutylation. See also * 1,1′-Bi-2-naphthol References {{DEFAULTSORT:Biphenol, 2, 2'- Phenols Biphenyls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphites
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy deriva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Halides
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existence of PI5.I. Tornieporth-Getting & T. Klapötke, ''J. Chem. Soc.'', ''Chem. Commun.'' 1990, 132. Mixed chalcogen halides also exist. Oxidation state +5 (PX5) In the gas phase the phosphorus pentahalides have trigonal bipyramidal molecular geometry as explained by VSEPR theory. Phosphorus pentafluoride is a relatively inert gas, notable as a mild Lewis acid and a fluoride ion acceptor. It is a fluxional molecule in which the axial (ax) and equatorial (eq) fluorine atoms interchange positions by the Berry pseudorotation mechanism. Phosphorus pentachloride, phosphorus pentabromide, and phosphorus heptabromide are ionic in the solid and liquid states; PCl5 is formulated as PCl4+PCl6–, but in contrast, PBr5 is formulated as PBr4+ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |