|
α-Methylbenzylamine
1-Phenylethylamine (1-PEA or α-PEA), also known as α-methylbenzylamine, is the organic compound with the formula C6H5CH(NH2)CH3. This primary amine is a colorless liquid is often used in chiral resolutions. Like benzylamine, it is relatively basic and forms stable ammonium salts and imines. Preparation and optical resolution 1-Phenylethylamine may be prepared by the reductive amination of acetophenone: : The Leuckart reaction, using ammonium formate, is another method for this transformation. -malic acid is used to resolve 1-Phenylethylamine, a versatile resolving agent in its own right. The dextrorotatory enantiomer crystallizes with the malate, leaving the levorotatory form in solution. Pharmacology Similarly to benzylamine and analogues like pargyline, 1-phenylethylamine has been found to act as a monoamine oxidase inhibitor (MAOI), as well as an inhibitor Inhibitor or inhibition may refer to: Biology * Enzyme inhibitor, a substance that binds to an enzyme and decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzylamines
Benzylamine, also known as phenylmethylamine, is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth. Manufacturing Benzylamine can be produced by several methods, the main industrial route being the reaction of benzyl chloride and ammonia. It is also produced by the reduction of benzonitrile and reductive amination of benzaldehyde, both done over Raney nickel. : It was first produced accidentally by Rudolf Leuckart in the reaction of benzaldehyde with formamide in a process now known as the Leuckart reaction. Bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzylamine
Benzylamine, also known as phenylmethylamine, is an Organic chemistry, organic chemical compound with the Structural formula#Condensed formulas, condensed structural formula C6H5CH2NH2 (sometimes abbreviated as Phenyl group#Nomenclature, PhCH2NH2 or Benzyl#Abbreviations, BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth. Manufacturing Benzylamine can be produced by several methods, the main industrial route being the reaction of benzyl chloride and ammonia. It is also produced by the reduction of benzonitrile and reductive amination of benzaldehyde, both done over Raney nickel. : It was first produced accidentally by Rudolf Leucka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superposable. Enantiomer molecules are like right and left hands: one cannot be superposed onto the other without first being converted to its mirror image. It is solely a relationship of chirality (chemistry), chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientation of a molecule as a whole or conformational isomerism, conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a ''racemic mixture'' or a ''racemate'', does not rotate light. Stereoisomers include both enantiomers and diastereomers. Diaste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Phenylethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation. Phenethylamine is sold as a dietary supplement for purported mood and weight loss-related therapeutic benefits; however, in orally ingested phenethylamine, a significant amount is metabolized in the small intestine by monoamine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semicarbazide-sensitive Amine Oxidase
Primary-amine oxidase, also known as semicarbazide-sensitive amine oxidase (SSAO), is an enzyme () with the systematic name ''primary-amine:oxygen oxidoreductase (deaminating)''. This enzyme catalyses the following chemical reaction : RCH2NH2 + H2O + O2 \rightleftharpoons RCHO + NH3 + H2O2 These enzymes are copper quinoproteins (2,4,5-trihydroxyphenylalanine quinone). Like monoamine oxidase (MAO), SSAO can deaminate short-chain primary amines, but is insensitive to MAO inhibitors. Semicarbazide inhibits the enzyme, in addition to other hydrazines, hydroxylamine and propargylamine. However, hydrazines are weak inhibitors and stronger inhibitors have been developed. SSAO is found in the smooth muscle of blood vessels and various other tissues. The physiological function of SSAO is not well understood. Development of blood vessels, lipolysis regulation, and detoxication are suggested. It may function as a scavenger enzyme to assist MAO. However, the oxidation process generates ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its Enzyme activity, activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which Substrate (biochemistry), substrate molecules are converted into Product (chemistry), products. An enzyme Enzyme catalysis, facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the Rate-determining step, most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind Reversible reaction, reversibly or irreversibly. Irreversible inhibitors form a Covalent bond, chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
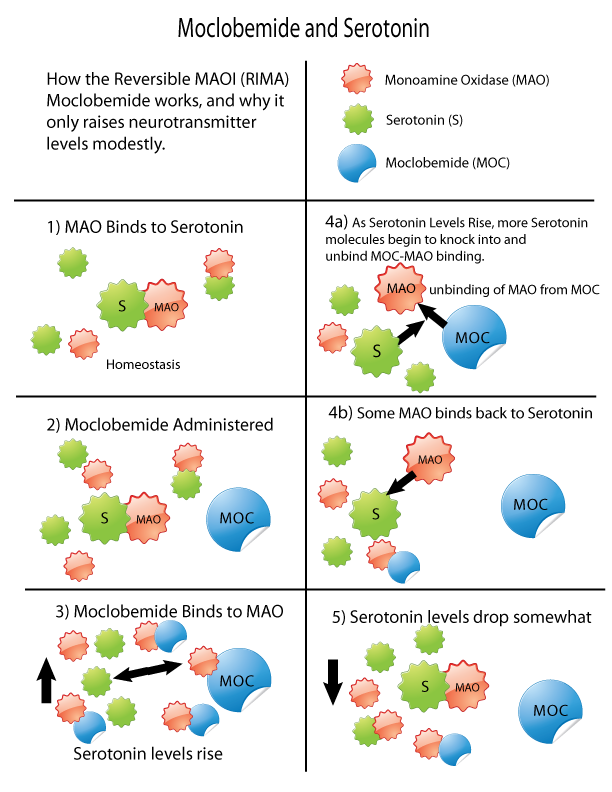
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that binding selectivity, selectively and Enzyme inhibitor#Reversible inhibitors, reversibly enzyme inhibitor, inhibit the MAO-A enzyme. RIMAs are used clinically in the medication, treatment of major depressive disorder, depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pargyline
Pargyline, sold under the brand name Eutonyl among others, is a monoamine oxidase inhibitor (MAOI) medication which has been used to treat hypertension (high blood pressure) but is no longer marketed. It has also been studied as an antidepressant, but was never licensed for use in the treatment of depression. The drug is taken by mouth. Side effects of pargyline include orthostatic hypotension among others. It has the potential for serious food and drug interactions with sympathomimetic agents like tyramine that can result in hypertensive crisis. Pargyline acts as a non-selective and irreversible inhibitor of the monoamine oxidases MAO-A and MAO-B. The exact mechanism of the hypotensive effects of pargyline and other MAOIs is unclear. Structurally, pargyline is a benzylamine derivative and is related to selegiline and clorgyline. Pargyline was first described in 1960 and was introduced for medical use in 1963. It was available in the United States and the United Kingdom. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Analog
A structural analog, also known as a chemical analog or simply an analog, is a chemical compound, compound having a chemical structure, structure similar to that of another compound, but differing from it in respect to a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced with other atoms, groups, or substructures. A structural analog can be imagined to be formed, at least theoretically, from the other compound. Structural analogs are often isoelectronicity, isoelectronic. Despite a high chemical similarity, structural analogs are not necessarily functional analog (chemistry), functional analogs and can have very different physical, chemical, biochemical, or pharmacological properties. In drug discovery, either a large series of structural analogs of an initial lead compound are created and tested as part of a structure–activity relationship study or a database is virtual screening, screened for structural analogs of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Resolution
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic mixture into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution. The use of chiral resolution to obtain enantiomerically pure compounds has the disadvantage of necessarily discarding at least half of the starting racemic mixture. Asymmetric synthesis of one of the enantiomers is one means of avoiding this waste. Crystallization of diastereomeric salts The most common method for chiral resolution involves conversion of the racemic mixture to a pair of diastereomeric derivatives by reacting them with chiral derivatizing agents, also known as chiral resolving agents. The derivatives which are then separated by conventional crystallization, and converted back to the enantiomers by removal of the resolving agent. The process can be laborious and depends on the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malic Acid
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is a metabolic intermediate in the citric acid cycle. Etymology The word 'malic' is derived from Latin , meaning 'apple'. The related Latin word , meaning 'apple tree', is used as the name of the genus ''Malus'', which includes all apples and crabapples; and is the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae. Biochemistry L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. File:L-Äpfelsäure.svg, L-Malic acid (''S'') File:D-Äpfelsäure.svg, D-Malic acid (''R'') Malate plays an important role i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |