|
Ψ-PEA (psychedelics)
Ψ-Phenethylamines (Ψ-PEA), or psi-phenethylamines (psi-PEA), also known as 4-substituted 2,6-dimethoxyphenethylamines, are a family of psychedelic and related compounds of the phenethylamine family. They are positional isomers of the 4-substituted 2,5-dimethoxyphenethylamines (e.g., 2Cs and DOx) and 4-substituted 3,5-dimethoxyphenethylamines (e.g., scalines and 3Cs). Like the preceding groups or substitution patterns of phenethylamine psychedelics, many Ψ-PEA derivatives are likewise potent serotonergic psychedelics and are known to act as serotonin 5-HT2A receptor agonists. Examples of known psychedelic Ψ-PEAs include TMA-6 (Ψ-TMA-2) and Ψ-DOM, Ψ-2C-T-4, Ψ-2C-DFMO, and Ψ-DODFMO. Conversely, Ψ-2C-O (TMPEA-6) was inactive. Unlike many other psychedelic phenethylamines, Ψ-PEAs such as TMA-6, Ψ-Aleph, and Ψ-Aleph-2 are known to act as potent monoamine oxidase inhibitors (MAOIs). The Ψ-PEAs are relatively unexplored compared to the other major psychedelic ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ψ-DOM
¤ê-DOM, or psi-DOM, also known as 2,6-dimethoxy-4-methylamphetamine, is a hallucinogenic, psychedelic drug of the ╬¿-PEA family and a structural isomer of the better-known hallucinogen DOM. ¤ê-DOM was first reported by Alexander Shulgin in his book ''PiHKAL''. ¤ê-DOM has similar effects to DOM, but is only around one third to one half the potency, with an active dose reported to be between 15 and 25 milligrams. The effects of ¤ê-DOM last for around six to eight hours. The activity of ¤ê-DOM (and ¤ê-2C-T-4) demonstrates that the two methoxy groups on the psychedelic phenethylamines are not strictly limited to the 2,5-positions on the phenyl ring. Indeed, any of the 2Cx or DOx series of drugs could alternatively be made as the 2,6-isomer and would still be expected to show similar activity, although slightly less potent. In theory this would vastly expand the range of different hallucinogens that could be derived from this family of drugs. The 2,6-isomer of another similar drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CT-5172
CT-5172, also known as 2,6-dimethoxy-3,5-dichlorophenethylamine, is a claimed hallucinogen of the phenethylamine family. It is an analogue of the serotonergic psychedelics mescaline and the 2C series but with an unusual substitution pattern on the benzene ring that includes methoxy groups at the 2 and 6 positions and chlorine atoms at the 3 and 5 positions. The drug was reported to have significant but relatively weak mescaline-like effects in cats. CT-5172 was first described in the scientific literature by 1969. Various related analogues, such as CT-5126 and CT-4719, have also been described. CT-5172 and related compounds were developed at the Laboratoire de Chimie Th├®rapeutique (CT; Therapeutic Chemistry Laboratory) of the Pasteur Institute in Paris, France. See also * ╬¿-2C-T-4 2C-T-4, also known as 4-isopropylthio-2,5-dimethoxyphenethylamine, is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and is used as entheogenic recre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Mescaline Analogue
A substituted mescaline analogue, also known as a scaline and typically but not always a 4-substituted 3,5-dimethoxyphenethylamine, is an analogue of the phenethylamine serotonergic psychedelic mescaline (3,4,5-trimethoxyphenethylamine). Other related compounds include the 2C (4-substituted 2,5-dimethoxyphenethylamine) and DOx (4-substituted 2,5-dimethoxyamphetamine) compounds as well as 3,4,5-trimethoxyamphetamine (TMA) and other 4-substituted 3,5-dimethoxyamphetamines (3C drugs). They are also mescaline analogues, but the 2C and DOx drugs have a third methoxy group in the 2 position instead of the 3 position while TMA is an amphetamine rather than a phenethylamine. The pharmacology of mescaline analogues has been studied. Mescaline analogues, or 4-substituted 3,5-dimethoxyphenethylamines specifically, tend to be much less potent than the 2C and DOx drugs. This relates to the fact that the 2,4,5-substitution pattern tends to be optimal in terms of receptor affinity and po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Methoxyphenethylamine
Methoxyphenethylamines (MPEAs), as well as methoxyamphetamines (MAs) in the case of the amphetamine (╬▒-methylphenethylamine) homologues, are substituted phenethylamines with one or more methoxy groups. In some cases, one or more of the methoxy groups may also be extended to form other alkoxy and related groups such as ethoxy or propoxy. Methoxyphenethylamines may have additional substitutions as well. Many methoxyphenethylamines that have multiple methoxy groups in the 2- through 5-positions of the phenyl ring, for instance mescaline, 2C-B, TMA, DOM, and 25I-NBOMe, are serotonin 5-HT2A receptor agonists and serotonergic psychedelics. Other methoxyphenethylamines, particularly monomethoxyamphetamines like ''para''-methoxyamphetamine (PMA), are monoamine releasing agents of serotonin, norepinephrine, and/or dopamine, with stimulant and/or entactogen-related effects. Compounds closely related to methoxyphenethylamines include methylenedioxyphenethylamines (MDxx) like M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TMPEA-6
2,4,6-Trimethoxyphenethylamine (2,4,6-TMPEA), also known as TMPEA-6, 2C-TMA-6, or ¤ê-2C-O, is a drug of the phenethylamine and ╬¿-PEA families. It is a positional isomer of mescaline (3,4,5-trimethoxyphenethylamine) and 2C-O (2,4,5-trimethoxyphenethylamine) as well as the ╬▒-desmethyl analogue of 2,4,6-trimethoxyamphetamine (TMA-6). Use and effects According to Daniel Trachsel in 2012, who cited personal communication with P. Rausch in 2009, the drug has been reported to be inactive in humans at a dose of up to 300mg or more. This is similar to the case of 2C-O (inactive at >300mg), but is in contrast to mescaline (active at ~180ÔÇô360mg) as well as TMA-6 (active at 25ÔÇô50mg). Pharmacology Unlike mescaline, but similarly to 2C-O, 2,4,6-TMPEA does not appear to be a substrate for amine oxidase. History 2,4,6-TMPEA was first described in the scientific literature by 1954. Alexander Shulgin mentioned 2,4,6-TMPEA in his 1991 book ''PiHKAL'' (''Phenethylamines I Have Known and Lov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
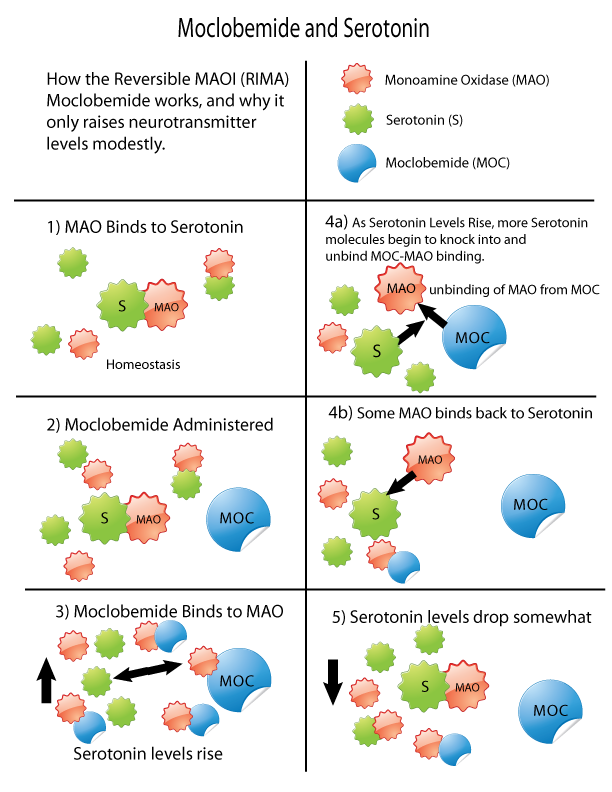
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that binding selectivity, selectively and Enzyme inhibitor#Reversible inhibitors, reversibly enzyme inhibitor, inhibit the MAO-A enzyme. RIMAs are used clinically in the medication, treatment of major depressive disorder, depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ψ-2C-O
2,4,6-Trimethoxyphenethylamine (2,4,6-TMPEA), also known as TMPEA-6, 2C-TMA-6, or ¤ê-2C-O, is a drug of the phenethylamine and ╬¿-PEA families. It is a positional isomer of mescaline (3,4,5-trimethoxyphenethylamine) and 2C-O (2,4,5-trimethoxyphenethylamine) as well as the ╬▒-desmethyl analogue of 2,4,6-trimethoxyamphetamine (TMA-6). Use and effects According to Daniel Trachsel in 2012, who cited personal communication with P. Rausch in 2009, the drug has been reported to be inactive in humans at a dose of up to 300mg or more. This is similar to the case of 2C-O (inactive at >300mg), but is in contrast to mescaline (active at ~180ÔÇô360mg) as well as TMA-6 (active at 25ÔÇô50mg). Pharmacology Unlike mescaline, but similarly to 2C-O, 2,4,6-TMPEA does not appear to be a substrate for amine oxidase. History 2,4,6-TMPEA was first described in the scientific literature by 1954. Alexander Shulgin mentioned 2,4,6-TMPEA in his 1991 book ''PiHKAL'' (''Phenethylamines I Have Known and Lov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |