|
Α-Halo Ketone
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone. Structure The general structure is RR′C(X)C(=O)R where R is an alkyl or aryl residue and X any one of the halogens. The preferred conformation of a halo ketone is that of a cisoid with the halogen and carbonyl sharing the same plane as the steric hindrance with the carbonyl alkyl group is generally larger. Halo ketone synthesis Halo ketones and halo carbonyl compounds in general are synthesized by reaction of carbonyl compounds with sources of X+ (X = halogen), which is provided using halogens: :RC(O)CH3 + X2 → RC(O)CH2X + HX Specialized sources of electrophilic halogenating agents include ''N''-Bromosuccinimide and 1,3-dibromo-5,5-dimethylhydantoin (DBDMH). In the Nierenstein reaction an acyl chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nierenstein Reaction
The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into a haloketone with diazomethane. It is an insertion reaction in that the methylene group from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride. Reaction mechanism The reaction proceeds through a diazonium salt intermediate formed when diazomethyl anion displaces the chloride: Excess diazomethane can act as a base, abstracting a hydrogen from the diazonium intermediate. The results are a neutral diazoketone, which does not react further; and methyldiazonium chloride, which decomposes to chloromethane. The unreactive diazoketone can, however, be re-activated with hydrogen chloride to give the Nierenstein product: In even some cases with limited diazomethane, the reaction process can stall into the diazoketone pathway, requiring reparative HCl gas. Examples Nierenstein's original 1924 publication: A reaction from benzoyl, benzoyl bromide going hay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tertiary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixture, racem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
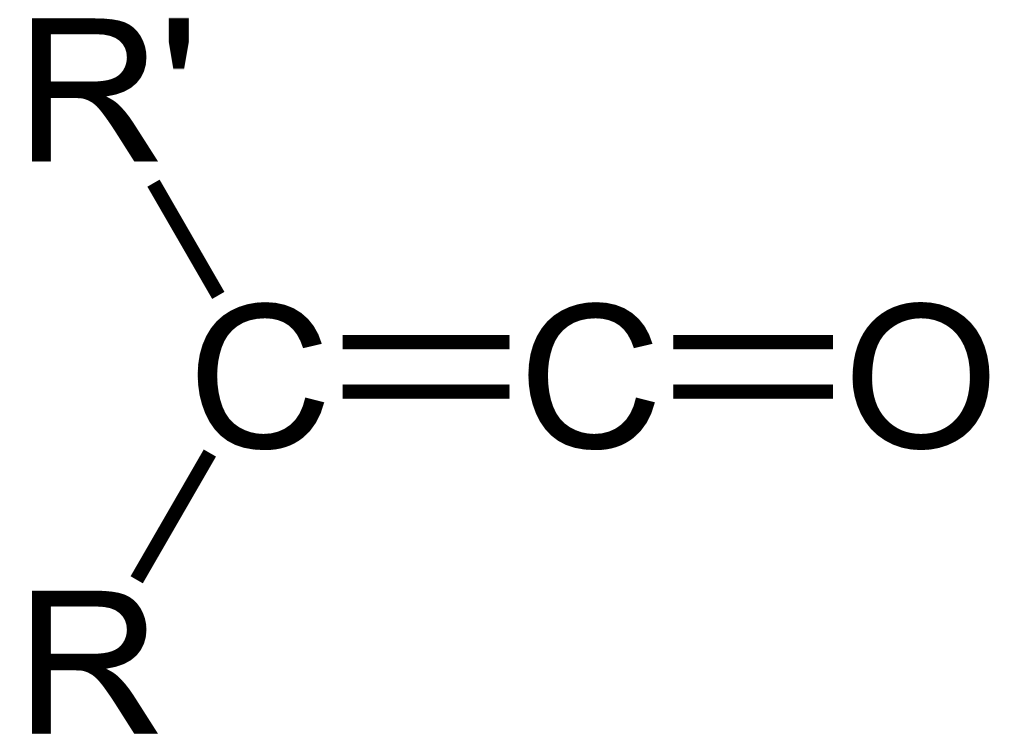
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Journal Of Organic Chemistry
''The Journal of Organic Chemistry'', colloquially known as ''JOC'', is a peer-reviewed scientific journal for original contributions of fundamental research in all branches of theory and practice in organic and bioorganic chemistry. It is published by the publishing arm of the American Chemical Society, with 24 issues per year. According to the ''Journal Citation Reports'', the journal had a 2023 impact factor of 3.3 and it is the journal that received the most cites (100,091 in 2017) in the field of organic chemistry. According to Web of Knowledge (and as December 2012), eleven papers from the journal have received more than 1,000 citations, with the most cited paper having received 7,967 citations. The current editor-in-chief is Scott J. Miller from Yale University. Indexing ''J. Org. Chem.'' is currently indexed in: See also * Organic Letters *Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also used as an ingredient in tonic water and other beverages to impart a bitter taste. Common side effects include headache, tinnitus, ringing in the ears, vision issues, and sweating. More severe side effects include deafness, thrombocytopenia, low blood platelets, and an irregular heartbeat. Use can make one more prone to sunburn. While it is unclear if use during pregnancy carries potential for fetal harm, treating malaria during pregnancy with quinine when appropriate is still recommended. Quinine is an alkaloid, a natural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, silane, germane, ammonia, and methane. It is an ionic material that is insoluble in all solvents (other than molten sodium metal), consistent with the fact that H− ions do not exist in solution. Basic properties and structure NaH is colorless, although samples generally appear grey. NaH is around 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H− centers in an octahedral geometry. The ionic radii of H− (146 pm in NaH) and F− (133 pm) are comparable, as judged by the Na−H and Na−F distances. "Inverse sodium hydride" (hydrogen sodide) A very unusual situation occurs in a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acid Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride Moiety (chemistry), moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : :Butyric acid, butyr''ic acid'' (C3H7COOH) → Butyryl chloride, butyr''yl chloride'' (C3H7COCl) (Idiosyncratically, for some trivial names, ''-oyl chloride'' substitutes ''-ic acid''. For example, pival''ic acid'' becomes pival''oyl chloride'' and acryl''ic acid'' becomes acryl''oyl chloride.'' The names pivalyl chloride and acrylyl chloride are less commonly used, although they are arguably more logical.) When other fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |