Nitrogenase on:
[Wikipedia]
[Google]
[Amazon]
Nitrogenases are

 While the mechanism for nitrogen fixation prior to the Janus E4 complex is generally agreed upon, there are currently two hypotheses for the exact pathway in the second half of the mechanism: the "distal" and the "alternating" pathway (see Figure 5). In the distal pathway, the terminal nitrogen is hydrogenated first, releases ammonia, then the nitrogen directly bound to the metal is hydrogenated. In the alternating pathway, one hydrogen is added to the terminal nitrogen, then one hydrogen is added to the nitrogen directly bound to the metal. This alternating pattern continues until ammonia is released. Because each pathway favors a unique set of intermediates, attempts to determine which path is correct have generally focused on the isolation of said intermediates, such as the nitrido in the distal pathway, and the
While the mechanism for nitrogen fixation prior to the Janus E4 complex is generally agreed upon, there are currently two hypotheses for the exact pathway in the second half of the mechanism: the "distal" and the "alternating" pathway (see Figure 5). In the distal pathway, the terminal nitrogen is hydrogenated first, releases ammonia, then the nitrogen directly bound to the metal is hydrogenated. In the alternating pathway, one hydrogen is added to the terminal nitrogen, then one hydrogen is added to the nitrogen directly bound to the metal. This alternating pattern continues until ammonia is released. Because each pathway favors a unique set of intermediates, attempts to determine which path is correct have generally focused on the isolation of said intermediates, such as the nitrido in the distal pathway, and the
 Binding of MgATP is one of the central events to occur in the mechanism employed by nitrogenase.
Binding of MgATP is one of the central events to occur in the mechanism employed by nitrogenase.
enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. ...
() that are produced by certain bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
, such as cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, bl ...
(blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
(N2) to ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
(NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecul ...
of molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
(nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
, amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide ''a''. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
There are two ...
.
Classification and structure
Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative enthalpy of reaction (), theactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
is very high (). Nitrogenase acts as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, reducing this energy barrier such that the reaction can take place at ambient temperatures.
A usual assembly consists of two components:
# The heterotetrameric
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer c ...
MoFe protein, a nitrogenase which uses the electrons provided to reduce N2 to NH3. In some assemblies it is replaced by a homologous alternative.
# The homodimeric Fe-only protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
, the reductase
A reductase is an enzyme that catalyzes a reduction reaction.
Examples
* 5α-Reductase
* 5β-Reductase
* Dihydrofolate reductase
* HMG-CoA reductase
* Methemoglobin reductase
* Ribonucleotide reductase
* Thioredoxin reductase
* ''E. coli' ...
which has a high reducing power and is responsible for the supply of electrons.
Reductase
The Fe protein, the dinitrogenase reductase or NifH, is a dimer of identical subunits which contains one e4S4cluster and has a mass of approximately 60-64kDa. The function of the Fe protein is to transfer electrons from areducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth met ...
, such as ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
or flavodoxin to the nitrogenase protein. The transfer of electrons requires an input of chemical energy which comes from the binding and hydrolysis of ATP. The hydrolysis of ATP also causes a conformational change within the nitrogenase complex, bringing the Fe protein and MoFe protein closer together for easier electron transfer.
Nitrogenase
The MoFe protein is a heterotetramer consisting of two α subunits and two β subunits, with a mass of approximately 240-250kDa. The MoFe protein also contains two iron–sulfur clusters, known as P-clusters, located at the interface between the α and β subunits and two FeMo cofactors, within the α subunits. The oxidation state of Mo in these nitrogenases was formerly thought Mo(V), but more recent evidence is for Mo(III). (Molybdenum in other enzymes is generally bound to molybdopterin as fully oxidized Mo(VI)). * The core (Fe8S7) of the P-cluster takes the form of two e4S3cubes linked by a central sulfur atom. Each P-cluster is linked to the MoFe protein by six cysteine residues. * Each FeMo cofactor (Fe7MoS9C) consists of two non-identical clusters: e4S3and oFe3S3 which are linked by three sulfide ions. Each FeMo cofactor is covalently linked to the α subunit of the protein by onecysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
residue and one histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the ...
residue.
Electrons from the Fe protein enter the MoFe protein at the P-clusters, which then transfer the electrons to the FeMo cofactors. Each FeMo cofactor then acts as a site for nitrogen fixation, with N2 binding in the central cavity of the cofactor.
Variations
The MoFe protein can be replaced by alternative nitrogenases in environments low in the Mo cofactor. Two types of such nitrogenases are known: the vanadium–iron (VFe; ''Vnf'') type and the iron–iron (FeFe; ''Anf'') type. Both form an assembly of two α subunits, two β subunits, and two δ (sometimes γ) subunits. The delta subunits are homologous to each other, and the alpha and beta subunits themselves are homologous to the ones found in MoFe nitrogenase. The gene clusters are also homologous, and these subunits are interchangeable to some degree. All nitrogenases use a similar Fe-S core cluster, and the variations come in the cofactor metal. The Anf nitrogenase in ''Azotobacter vinelandii'' is organized in an ''anfHDGKOR'' operon. This operon still requires some of the Nif genes to function. An engineered minimal 10-gene operon that incorporates these additional essential genes has been constructed.Mechanism
General mechanism
Nitrogenase is an enzyme responsible for catalyzingnitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
, which is the reduction of nitrogen (N2) to ammonia (NH3) and a process vital to sustaining life on Earth. There are three types of nitrogenase found in various nitrogen-fixing bacteria: molybdenum (Mo) nitrogenase, vanadium (V) nitrogenase, and iron-only (Fe) nitrogenase. Molybdenum nitrogenase, which can be found in diazotroph Diazotrophs are bacteria and archaea that fix gaseous nitrogen in the atmosphere into a more usable form such as ammonia.
A diazotroph is a microorganism that is able to grow without external sources of fixed nitrogen. Examples of organisms tha ...
s such as legume
A legume () is a plant in the family Fabaceae (or Leguminosae), or the fruit or seed of such a plant. When used as a dry grain, the seed is also called a pulse. Legumes are grown agriculturally, primarily for human consumption, for livestock for ...
-associated rhizobia
Rhizobia are diazotrophic bacteria that fix nitrogen after becoming established inside the root nodules of legumes (Fabaceae). To express genes for nitrogen fixation, rhizobia require a plant host; they cannot independently fix nitrogen. In g ...
, is the nitrogenase that has been studied the most extensively and thus is the most well characterized. Figures 1-2 display the crystal structure and key catalytic components of molybdenum nitrogenase extracted from ''Azotobacter vinelandii''. Vanadium nitrogenase and iron-only nitrogenase can both be found in select species of Azotobacter as an alternative nitrogenase. Equations 1 and 2 show the balanced reactions of nitrogen fixation in molybdenum nitrogenase and vanadium nitrogenase respectively.
All nitrogenases are two-component systems made up of Component I (also known as dinitrogenase) and Component II (also known as dinitrogenase reductase). Component I is a MoFe protein in molybdenum nitrogenase, a VFe protein in vanadium nitrogenase, and a Fe protein in iron-only nitrogenase. Component II is a Fe protein that contains the Fe-S cluster (Figure 3: top), which transfers electrons to Component I. Component I contains 2 key metal clusters: the P-cluster (Figure 3: middle), and the FeMo-cofactor (FeMo-co) (Figure 3: bottom). Mo is replaced by V or Fe in vanadium nitrogenase and iron-only nitrogenase respectively. During catalysis, electrons flow from a pair of ATP molecules within Component II to the Fe-S cluster, to the P-cluster, and finally to the FeMo-co, where reduction of N2 to NH3 takes place.
Lowe-Thorneley kinetic model
The reduction of nitrogen to two molecules of ammonia is carried out at the FeMo-co of Component I after the sequential addition of proton and electron equivalents from Component II.Steady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
, freeze quench, and stopped-flow kinetics measurements carried out in the 70's and 80's by Lowe, Thorneley, and others provided a kinetic basis for this process. The Lowe-Thorneley (LT) kinetic model (depicted in Figure 4) was developed from these experiments and documents the eight correlated proton and electron transfers required throughout the reaction. Each intermediate stage is depicted as En where n = 0-8, corresponding to the number of equivalents transferred. The transfer of four equivalents are required before the productive addition of N2, although reaction of E3 with N2 is also possible. Notably, nitrogen reduction has been shown to require 8 equivalents of protons and electrons as opposed to the 6 equivalents predicted by the balanced chemical reaction.
Intermediates E0 through E4
Spectroscopic characterization of these intermediates has allowed for greater understanding of nitrogen reduction by nitrogenase, however, the mechanism remains an active area of research and debate. Briefly listed below are spectroscopic experiments for the intermediates before the addition of nitrogen: E0 – This is the resting state of the enzyme before catalysis begins. EPR characterization shows that this species has a spin of 3/2. E1 – The one electron reduced intermediate has been trapped during turnover under N2. Mӧssbauer spectroscopy of the trapped intermediate indicates that the FeMo-co is integer spin greater than 1. E2 – This intermediate is proposed to contain the metal cluster in its resting oxidation state with the two added electrons stored in a bridginghydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
and the additional proton bonded to a sulfur atom. Isolation of this intermediate in mutated enzymes shows that the FeMo-co is high spin and has a spin of 3/2.
E3 – This intermediate is proposed to be the singly reduced FeMo-co with one bridging hydride and one proton.
E4 – Termed the Janus intermediate after the Roman god of transitions, this intermediate is positioned after exactly half of the electron proton transfers and can either decay back to E0 or proceed with nitrogen binding and finish the catalytic cycle. This intermediate is proposed to contain the FeMo-co in its resting oxidation state with two bridging hydrides and two sulfur bonded protons. This intermediate was first observed using freeze quench techniques with a mutated protein in which residue 70, a valine amino acid, is replaced with isoleucine. This modification prevents substrate access to the FeMo-co. EPR characterization of this isolated intermediate shows a new species with a spin of ½. ENDOR Endor or Ein Dor may refer to:
Places
* Endor (village), from the Hebrew Bible, a Canaanite village where the Witch of Endor lived
* Indur, a Palestinian village depopulated during the 1948 Arab-Israeli war
* Ein Dor, a Kibbutz in modern Israel
F ...
experiments have provided insight into the structure of this intermediate, revealing the presence of two bridging hydrides. 95Mo and 57Fe ENDOR Endor or Ein Dor may refer to:
Places
* Endor (village), from the Hebrew Bible, a Canaanite village where the Witch of Endor lived
* Indur, a Palestinian village depopulated during the 1948 Arab-Israeli war
* Ein Dor, a Kibbutz in modern Israel
F ...
show that the hydrides bridge between two iron centers. Cryoannealing of the trapped intermediate at -20 °C results in the successive loss of two hydrogen equivalents upon relaxation, proving that the isolated intermediate is consistent with the E4 state. The decay of E4 to E2 + H2 and finally to E0 and 2H2 has confirmed the EPR signal associated with the E2 intermediate.
The above intermediates suggest that the metal cluster is cycled between its original oxidation state and a singly reduced state with additional electrons being stored in hydrides. It has alternatively been proposed that each step involves the formation of a hydride and that the metal cluster actually cycles between the original oxidation state and a singly oxidized state.
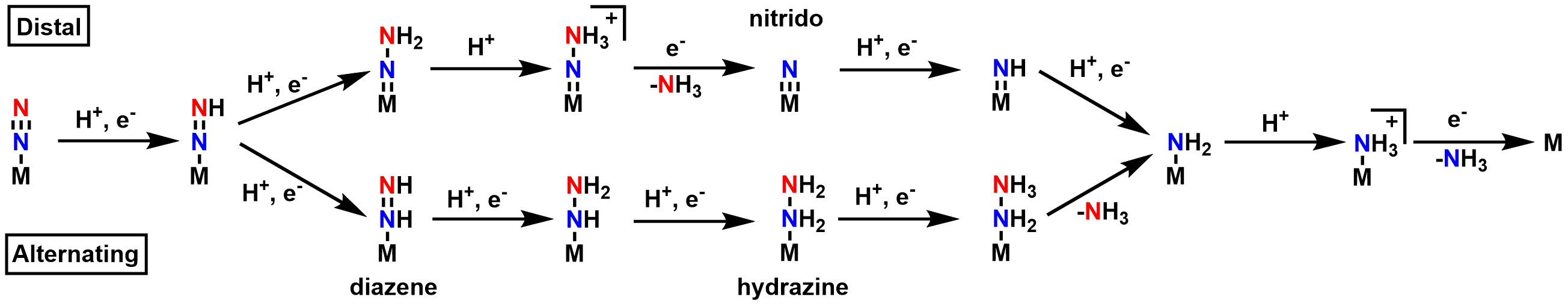
Distal and alternating pathways for N2 fixation
 While the mechanism for nitrogen fixation prior to the Janus E4 complex is generally agreed upon, there are currently two hypotheses for the exact pathway in the second half of the mechanism: the "distal" and the "alternating" pathway (see Figure 5). In the distal pathway, the terminal nitrogen is hydrogenated first, releases ammonia, then the nitrogen directly bound to the metal is hydrogenated. In the alternating pathway, one hydrogen is added to the terminal nitrogen, then one hydrogen is added to the nitrogen directly bound to the metal. This alternating pattern continues until ammonia is released. Because each pathway favors a unique set of intermediates, attempts to determine which path is correct have generally focused on the isolation of said intermediates, such as the nitrido in the distal pathway, and the
While the mechanism for nitrogen fixation prior to the Janus E4 complex is generally agreed upon, there are currently two hypotheses for the exact pathway in the second half of the mechanism: the "distal" and the "alternating" pathway (see Figure 5). In the distal pathway, the terminal nitrogen is hydrogenated first, releases ammonia, then the nitrogen directly bound to the metal is hydrogenated. In the alternating pathway, one hydrogen is added to the terminal nitrogen, then one hydrogen is added to the nitrogen directly bound to the metal. This alternating pattern continues until ammonia is released. Because each pathway favors a unique set of intermediates, attempts to determine which path is correct have generally focused on the isolation of said intermediates, such as the nitrido in the distal pathway, and the diazene
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is ...
and hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
in the alternating pathway. Attempts to isolate the intermediates in nitrogenase itself have so far been unsuccessful, but the use of model complexes has allowed for the isolation of intermediates that support both sides depending on the metal center used. Studies with Mo generally point towards a distal pathway, while studies with Fe generally point towards an alternating pathway.
Specific support for the distal pathway has mainly stemmed from the work of Schrock and Chatt, who successfully isolated the nitrido complex using Mo as the metal center in a model complex. Specific support for the alternating pathway stems from a few studies. Iron only model clusters have been shown to catalytically reduce N2. Small tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
clusters have also been shown to follow an alternating pathway for nitrogen fixation. The vanadium nitrogenase releases hydrazine, an intermediate specific to the alternating mechanism. However, the lack of characterized intermediates in the native enzyme itself means that neither pathway has been definitively proven. Furthermore, computational studies have been found to support both sides, depending on whether the reaction site is assumed to be at Mo (distal) or at Fe (alternating) in the MoFe cofactor.
Mechanism of MgATP binding
 Binding of MgATP is one of the central events to occur in the mechanism employed by nitrogenase.
Binding of MgATP is one of the central events to occur in the mechanism employed by nitrogenase. Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of the terminal phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
group of MgATP provides the energy needed to transfer electrons from the Fe protein to the MoFe protein. The binding interactions between the MgATP phosphate groups and the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
residues of the Fe protein are well understood by comparing to similar enzymes, while the interactions with the rest of the molecule are more elusive due to the lack of a Fe protein crystal structure with MgATP bound (as of 1996). Three protein residues have been shown to have significant interactions with the phosphates, shown in Figure 6. In the absence of MgATP, a salt bridge exists between residue 15, lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated − ...
, and residue 125, aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
. Upon binding, this salt bridge is interrupted. Site-specific mutagenesis has demonstrated that when the lysine is substituted for a glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
, the protein's affinity for MgATP is greatly reduced and when the lysine is substituted for an arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the am ...
, MgATP cannot bind due to the salt bridge being too strong. The necessity of specifically aspartic acid at site 125 has been shown through noting altered reactivity upon mutation of this residue to glutamic acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synt ...
. The third residue that has been shown to be key for MgATP binding is residue 16, serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − for ...
. Site-specific mutagenesis was used to demonstrate this fact. This has led to a model in which the serine remains coordinated to the Mg2+ ion after phosphate hydrolysis in order to facilitate its association with a different phosphate of the now ADP molecule. MgATP binding also induces significant conformational changes within the Fe protein. Site-directed mutagenesis was employed to create mutants in which MgATP binds but does not induce a conformational change. Comparing X-ray scattering data in the mutants versus in the wild-type protein led to the conclusion that the entire protein contracts upon MgATP binding, with a decrease in radius of approximately 2.0 Å.
Other mechanistic details
Many mechanistic aspects ofcatalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
remain unknown. No crystallographic analysis has been reported on substrate bound to nitrogenase.
Nitrogenase is able to reduce acetylene, but is inhibited by carbon monoxide, which binds to the enzyme and thereby prevents binding of dinitrogen. Dinitrogen prevent acetylene binding, but acetylene does not inhibit binding of dinitrogen and requires only one electron for reduction to ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
. Due to the oxidative properties of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
, most nitrogenases are irreversibly inhibited by dioxygen, which degradatively oxidizes the Fe-S cofactors. This requires mechanisms for nitrogen fixers to protect nitrogenase from oxygen ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and p ...
''. Despite this problem, many use oxygen as a terminal electron acceptor for respiration. Although the ability of some nitrogen fixers such as Azotobacteraceae to employ an oxygen-labile nitrogenase under aerobic conditions has been attributed to a high metabolic rate
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
, allowing oxygen reduction at the cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
, the effectiveness of such a mechanism has been questioned at oxygen concentrations above 70 μM (ambient concentration is 230 μM O2), as well as during additional nutrient limitations.
Nonspecific reactions
In addition to dinitrogen reduction, nitrogenases also reduceprotons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron m ...
to dihydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
, meaning nitrogenase is also a dehydrogenase
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as ...
. A list of other reactions carried out by nitrogenases is shown below:
: HC≡CH → H2C=CH2
: N–=N+=O → N2 + H2O
: N=N=N– → N2 + NH3
: → CH4, NH3, H3C–CH3, H2C=CH2 (CH3NH2)
:N≡C–R → RCH3 + NH3
:C≡N–R → CH4, H3C–CH3, H2C=CH2, C3H8, C3H6, RNH2
: O=C=S → CO + H2S
: O=C=O → CO + H2O
: S=C=N– → H2S + HCN
: O=C=N– → H2O + HCN, CO + NH3
Furthermore, dihydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
functions as a competitive inhibitor, carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
functions as a non-competitive inhibitor, and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
functions as a rapid-equilibrium inhibitor of nitrogenase.
Vanadium nitrogenases have also been shown to catalyze the conversion of CO into alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in ...
through a reaction comparable to Fischer-Tropsch synthesis.
Organisms that synthesize nitrogenase
There are two types of bacteria that synthesize nitrogenase and are required for nitrogen fixation. These are: * Free-living bacteria (non-symbiotic
Symbiosis (from Greek , , "living together", from , , "together", and , bíōsis, "living") is any type of a close and long-term biological interaction between two different biological organisms, be it mutualistic, commensalistic, or para ...
), examples include:
** Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, bl ...
(blue-green algae)
** Green sulfur bacteria
The green sulfur bacteria are a phylum of obligately anaerobic photoautotrophic bacteria that metabolize sulfur.
Green sulfur bacteria are nonmotile (except ''Chloroherpeton thalassium'', which may glide) and capable of anoxygenic photosynthe ...
** ''Azotobacter
''Azotobacter'' is a genus of usually motile, oval or spherical bacteria that form thick-walled cysts (and also has hard crust) and may produce large quantities of capsular slime. They are aerobic, free-living soil microbes that play an impo ...
''
* Mutualistic bacteria (symbiotic), examples include:
** ''Rhizobium
''Rhizobium'' is a genus of Gram-negative soil bacteria that fix nitrogen. ''Rhizobium'' species form an endosymbiotic nitrogen-fixing association with roots of (primarily) legumes and other flowering plants.
The bacteria colonize plant cells ...
'', associated with leguminous plants
** ''Spirillum
''Spirillum'' is a genus of Gram-negative bacteria in the family '' Spirillaceae'' of the '' Nitrosomonadales'' of the '' Betaproteobacteria''.Garrity, George M.; Brenner, Don J.; Krieg, Noel R.; Staley, James T. (eds.) (2005). Bergey's Manual ...
'', associated with cereal
A cereal is any grass cultivated for the edible components of its grain (botanically, a type of fruit called a caryopsis), composed of the endosperm, germ, and bran. Cereal grain crops are grown in greater quantities and provide more food ...
grasses
** '' Frankia''
Similarity to other proteins
The three subunits of nitrogenase exhibit significant sequence similarity to three subunits of the light-independent version ofprotochlorophyllide reductase
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide ''a''. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
There are two ...
that performs the conversion of protochlorophyllide
Protochlorophyllide,KEGG compound database entr/ref> or monovinyl protochlorophyllide, is an intermediate in the biosynthesis of chlorophyll ''a''. It lacks the phytol side-chain of chlorophyll and the reduced pyrrole in ring D. Protochlorophy ...
to chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to ...
. This protein is present in gymnosperms, algae, and photosynthetic bacteria but has been lost by angiosperms during evolution.
Separately, two of the nitrogenase subunits (NifD and NifH) have homologues in methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are c ...
s that do not fix nitrogen e.g. ''Methanocaldococcus jannaschii
''Methanocaldococcus jannaschii'' (formerly ''Methanococcus jannaschii'') is a thermophilic methanogenic archaean in the class Methanococci. It was the first archaeon to have its complete genome sequenced. The sequencing identified many genes un ...
''. Little is understood about the function of these "class IV" ''nif'' genes, though they occur in many methanogens. In ''M. jannaschii'' they are known to interact with each other and are constitutively expressed.
Measurement of nitrogenase activity
As with many assays for enzyme activity, it is possible to estimate nitrogenase activity by measuring the rate of conversion of the substrate (N2) to the product (NH3). Since NH3 is involved in other reactions in the cell, it is often desirable to label the substrate with 15N to provide accounting or "mass balance" of the added substrate. A more common assay, the acetylene reduction assay or ARA, estimates the activity of nitrogenase by taking advantage of the ability of the enzyme to reduce acetylene gas to ethylene gas. These gases are easily quantified using gas chromatography. Though first used in a laboratory setting to measure nitrogenase activity in extracts of ''Clostridium pasteurianum
''Clostridium pasteurianum'' (previously known as ''Clostridium pastorianum'') is a bacterium discovered in 1890 by the Russian microbiologist Sergei Winogradsky. It was the first free living (non-symbiotic) micro-organism discovered that could ...
'' cells, ARA has been applied to a wide range of test systems, including field studies where other techniques are difficult to deploy. For example, ARA was used successfully to demonstrate that bacteria associated with rice roots undergo seasonal and diurnal rhythms in nitrogenase activity, which were apparently controlled by the plant.
Unfortunately, the conversion of data from nitrogenase assays to actual moles of N2 reduced (particularly in the case of ARA), is not always straightforward and may either underestimate or overestimate the true rate for a variety of reasons. For example, H2 competes with N2 but not acetylene for nitrogenase (leading to overestimates of nitrogenase by ARA). Bottle or chamber-based assays may produce negative impacts on microbial systems as a result of containment or disruption of the microenvironment through handling, leading to underestimation of nitrogenase. Despite these weaknesses, such assays are very useful in assessing relative rates or temporal patterns in nitrogenase activity.
See also
*Nitrogen fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmo ...
* Abiological nitrogen fixation
Abiological nitrogen fixation describes chemical processes that fix (react with) N2, usually with the goal of generating ammonia. The dominant technology for abiological nitrogen fixation is the Haber process, which uses an iron-based heterogeneou ...
References
Further reading
*External links
* {{Portal bar, Biology, border=no EC 1.18.6 Iron–sulfur proteins Nitrogen cycle Molybdenum enzymes