|
Iron–sulfur Cluster
Iron–sulfur clusters (or iron–sulphur clusters in British spelling) are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe–S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters (see Figure). It is believed that the last universal common ancestor had many iron-sulfur clusters. Organometallic clusters Organometallic Fe–S clusters include the sulfido carbonyls with the formula Fe2S2(CO)6, H2Fe3S(CO)9, and Fe3S2(CO)9. Compounds are also known that incorporate cyclopentadienyl ligands, such as (C5H5)4Fe4S4. Inorganic materials center, Structure of potassium dithioferrate, which features infinite chains of Fe(III) centers. Biological Fe–S clusters Iron–sulfur clusters occur in many biological systems, often as components of electron transfer proteins. The ferredoxin proteins a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rieske Protein
Rieske proteins are iron–sulfur protein (ISP) components of cytochrome ''bc''1 complexes and cytochrome b6f complexes and are responsible for electron transfer in some biological systems. John S. Rieske and co-workers first discovered the protein and in 1964 isolated an acetylated form of the bovine mitochondrial protein. In 1979 Trumpower's lab isolated the "oxidation factor" from bovine mitochondria and showed it was a reconstitutively-active form of the Rieske iron-sulfur protein It is a unique Fe-2Scluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV. Biological function Ubiquinol-cytochrome-c reductase (also known as bc1 complex or complex III) is an enzyme complex of bacterial and mitochondrial oxidative phosphorylation systems. It catalyses the oxidation-reduction reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Chemistry
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster'' may be used for ensembles with up to couple dozen atoms. Clusters with a definite number and type of atoms in a specific arrangement are often considered a specific chemical compound and are studied as such. For example, fullerene is a cluster of 60 carbon atoms arranged as the vertices of a truncated icosahedron, and decaborane is a cluster of 10 boron atoms forming an incomplete icosahedron, surrounded by 14 hydrogen atoms. The term is most commonly used for ensembles consisting of several atoms of the same element, or of a few different elements, bonded in a three-dimensional arrangement. Transition metals and main group elements form especially robust clusters. Indeed, in some contexts, the term may refer specifically to a met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioinorganic Chemistry
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins. As a mix of biochemistry and inorganic chemistry, bioinorganic chemistry is important in elucidating the implications of electron-transfer proteins, substrate bindings and activation, atom and group transfer chemistry as well as metal properties in biological chemistry. The successful development of truly interdisciplinary work is necessary to advance bioinorganic chemistry. Composition of living organisms About 99% of mammals' mass are the elements car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transport Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Sericytochromatia, the proposed name of the paraphyletic and most basal group, is the ancestor of both the non-photosynthetic group Melainabacteria and the photosynthetic cyanobacteria, also called Oxyphotobacteria. Cyanobacteria use photosynthetic pigments, such as carotenoids, phycobilins, and various forms of chlorophyll, which absorb energy from light. Unlike heterotrophic prokaryotes, cyanobacteria have internal membranes. These are flattened sacs called thylakoids where photosynthesis is performed. Phototrophic eukaryotes such as green plants perform photosynthesis in pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome B6f Complex
The cytochrome ''b''6''f'' complex (plastoquinol—plastocyanin reductase; ) is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, that catalyzes the transfer of electrons from plastoquinol to plastocyanin. The reaction is analogous to the reaction catalyzed by cytochrome bc1 (Complex III) of the mitochondrial electron transport chain. During photosynthesis, the cytochrome b6f complex is one step along the chain that transfers electrons from Photosystem II to Photosystem I, and at the same time pumps protons into the thylakoid space, contributing to the generation of an electrochemical (energy) gradient that is later used to synthesize ATP from ADP. Enzyme structure The cytochrome b6f complex is a dimer, with each monomer composed of eight subunits. These consist of four large subunits: a 32 kDa cytochrome f with a c-type cytochrome, a 25 kDa cytochrome b6 with a low- and high-potential heme group, a 19 kDa Rieske ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like '' Arabidopsis'' and wheat. A chloroplast is characterized by its two membranes and a high concentration of chlorophyll. Other plastid types, such as the leucoplast and the chromoplast, contain little chlorophyll and do not carry out photosynthesis. Chloroplasts are highly dynamic—they cir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
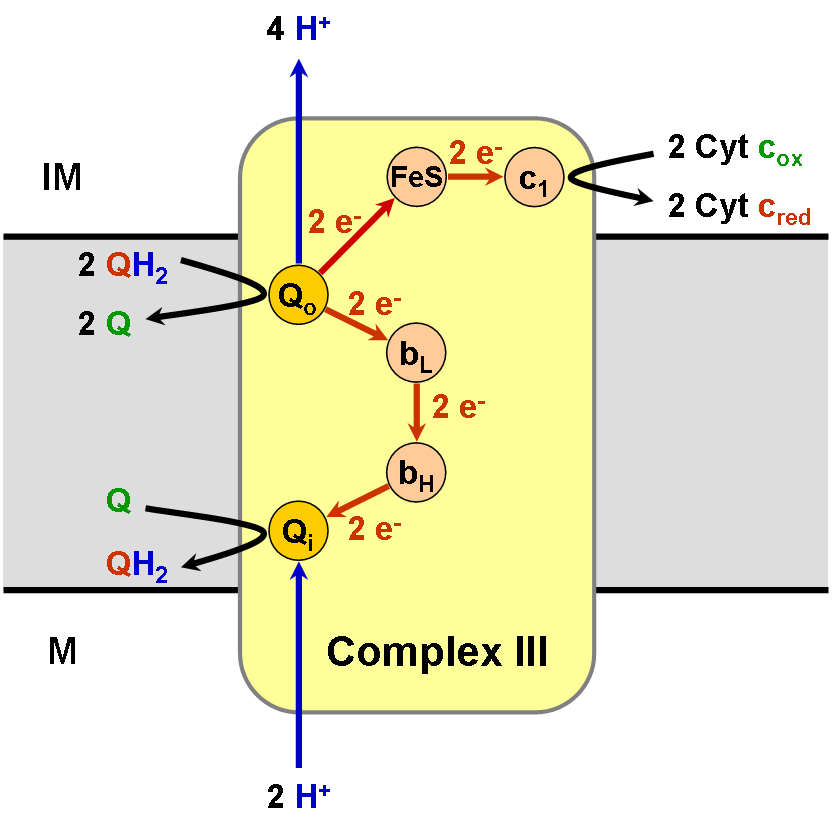
Coenzyme Q – Cytochrome C Reductase
The coenzyme Q : cytochrome ''c'' – oxidoreductase, sometimes called the cytochrome ''bc''1 complex, and at other times complex III, is the third complex in the electron transport chain (), playing a critical role in biochemical generation of ATP (oxidative phosphorylation). Complex III is a multisubunit transmembrane protein encoded by both the mitochondrial ( cytochrome b) and the nuclear genomes (all other subunits). Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders. The bc1 complex contains 11 subunits, 3 respiratory subunits (cytochrome B, cytochrome C1, Rieske protein), 2 core proteins and 6 low-molecular weight proteins. Ubiquinol—cytochrome-c reductase catalyzes the chemical reaction :QH2 + 2 ferricytochrome c \rightleftharpoons Q + 2 ferrocytochrome c + 2 H+ Thus, the two substrates of this enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Potential Iron–sulfur Protein
High potential iron-sulfur proteins (HIPIP) are a specific class of high-redox potential 4Fe-4S ferredoxins that functions in anaerobic electron transport and which occurs in photosynthetic bacteria and in '' Paracoccus denitrificans''. The HiPIPs are small proteins which show significant variation in their sequences, their sizes (from 63 to 85 amino acids), and in their oxidation- reduction potentials. As shown in the following schematic representation the iron-sulfur cluster is bound by four conserved cysteine residues. 4Fe-4S cluster , , , , xxxxxxxxxxxxxxxxxxxCxCxxxxxxxCxxxxxCxxxx C: conserved cysteine involved in the binding of the iron-sulfur cluster. e4S4clusters The e4S4clusters are abundant cofactors of metalloproteins. They participate in electron-transfer sequences. The core structure for the e4S4cluster is a cube with alternating Fe and S vertices. These clusters exist in two oxidation st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
British English
British English (BrE, en-GB, or BE) is, according to Lexico, Oxford Dictionaries, "English language, English as used in Great Britain, as distinct from that used elsewhere". More narrowly, it can refer specifically to the English language in England, or, more broadly, to the collective dialects of English throughout the British Isles taken as a single umbrella variety, for instance additionally incorporating Scottish English, Welsh English, and Ulster English, Northern Irish English. Tom McArthur (linguist), Tom McArthur in the ''Oxford Guide to World English'' acknowledges that British English shares "all the ambiguities and tensions [with] the word 'British people, British' and as a result can be used and interpreted in two ways, more broadly or more narrowly, within a range of blurring and ambiguity". Variations exist in formal (both written and spoken) English in the United Kingdom. For example, the adjective ''wee'' is almost exclusively used in parts of Scotland, North E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium '' Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight- excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters. These biological "capacitors" can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_cyt6bf_mutant.jpg)