Hapticity on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 Here one of the η6-benzene rings changes to a η4-benzene.
Similarly hapticity can change during a substitution reaction:
:
Here one of the η6-benzene rings changes to a η4-benzene.
Similarly hapticity can change during a substitution reaction:
: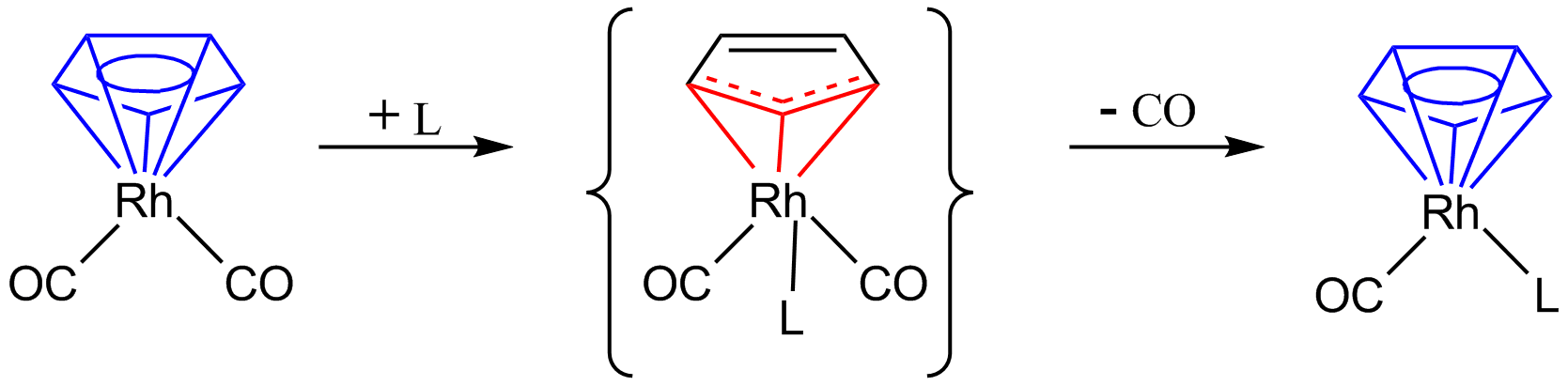 Here the η5-cyclopentadienyl changes to an η3-cyclopentadienyl, giving room on the metal for an extra 2-electron donating ligand 'L'. Removal of one molecule of CO and again donation of two more electrons by the cyclopentadienyl ligand restores the η5-cyclopentadienyl. The so-called
Here the η5-cyclopentadienyl changes to an η3-cyclopentadienyl, giving room on the metal for an extra 2-electron donating ligand 'L'. Removal of one molecule of CO and again donation of two more electrons by the cyclopentadienyl ligand restores the η5-cyclopentadienyl. The so-called
coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
, hapticity is the coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a cent ...
of a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
to a metal center via an uninterrupted and contiguous series of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
s. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
s.
History
The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Orgel, and Rich described the structure of the "sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
" ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
where an iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
atom is ''"sandwiched"'' between two parallel cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
rings. Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus '' Gossypium'' in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor pe ...
later proposed the term ''hapticity'' derived from the adjectival prefix hapto (from the Greek ''haptein'', to fasten, denoting contact or combination) placed before the name of the olefin, where the Greek letter η (eta) is used to denote the number of contiguous atoms of a ligand that bind to a metal center. The term is usually employed to refer to ligands containing extended π-systems or where agostic bonding is not obvious from the formula.
Historically important compounds where the ligands are described with hapticity
*Ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
: bis(η5-cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
)iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
* Uranocene: bis(η8-1,3,5,7- cyclooctatetraene)uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
*W(CO)3(PPri3)2(η2-H2): the first compound to be synthesized with a dihydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
ligand.
*IrCl(CO) (C6H5)3sub>2(η2-O2): the dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
derivative which forms reversibly upon oxygenation of Vaska's complex.
Examples
The η-notation is encountered in many coordination compounds: *Side-on bonding of molecules containing σ-bonds like H2: **W(CO)3(PiPr3)2(η2-H2) *Side-on bonded ligands containing multiple bonded atoms, e.g.ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
in Zeise's salt or with fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
, which is bonded through donation of the π-bonding electrons:
**K tCl3(η2-C2H4)sup>. H2O
*Related complexes containing bridging π-ligands:
**(μ-η2:η2- C2H2)Co2(CO)6 and ( Cp*2 Sm)2(μ-η2:η2- N2)
**Dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
in bis(μ-η2:η2-O2),
::Note that with some bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
s, an alternative bridging mode is observed, e.g. κ1,κ1, like in (Me3SiCH2)3V(μ-N2-κ1(N),κ1(N′))V(CH2SiMe3)3 contains a bridging dinitrogen molecule, where the molecule is end-on coordinated to the two metal centers (see hapticity vs. denticity).
*The bonding of π-bonded species can be extended over several atoms, e.g. in allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
, butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two v ...
ligands, but also in cyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
or benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
rings can share their electrons.
*Apparent violations of the 18-electron rule sometimes are explicable in compounds with unusual hapticities:
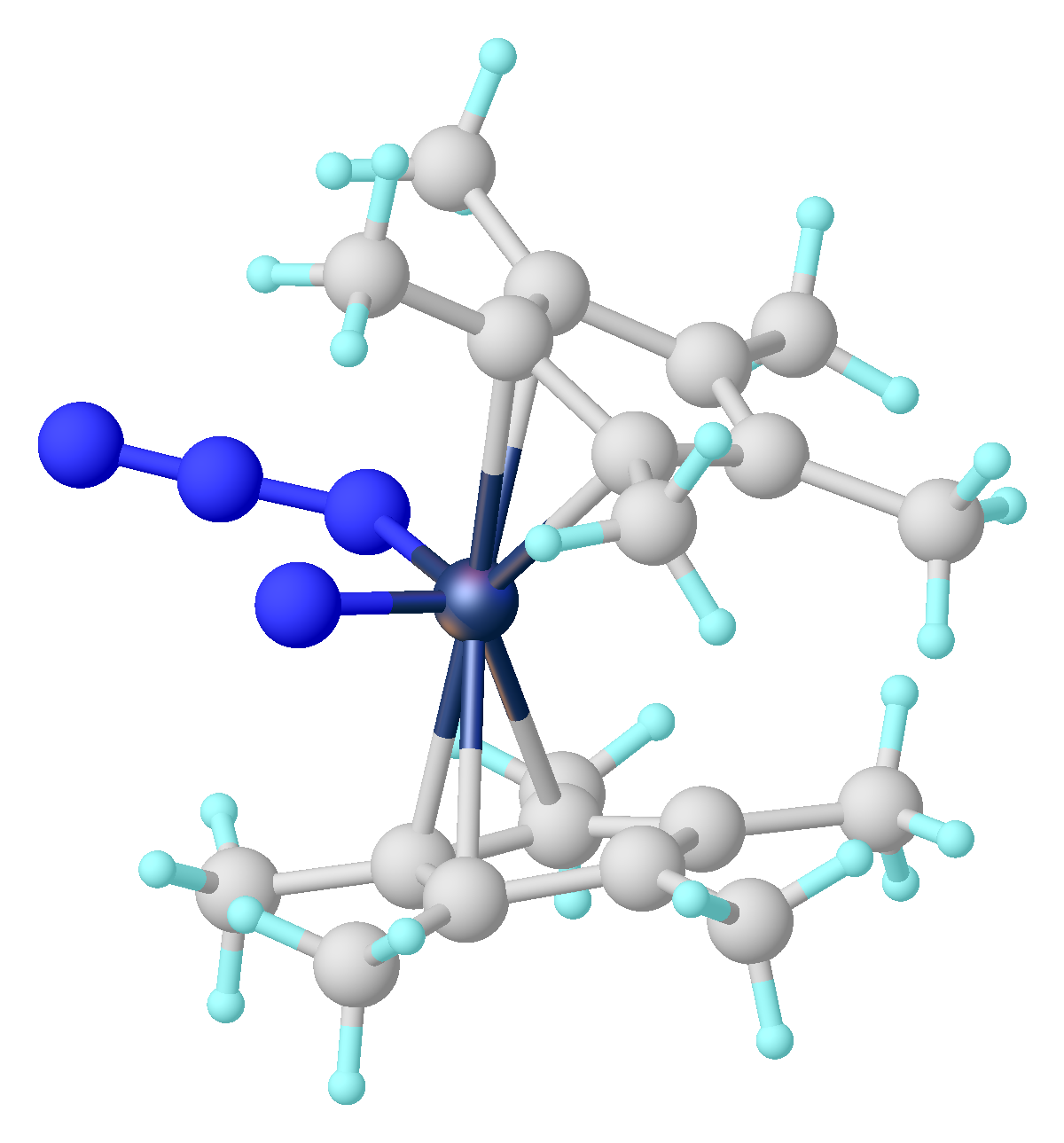
**The 18-VE complex (η5-C5H5)Fe(η1-C5H5)(CO)2 contains one η5 bonded cyclopentadienyl, and one η1 bonded cyclopentadienyl.
** Reduction of the 18-VE compound u(η6-C6Me6)2sup>2+ (where both aromatic rings are bonded in an η6-coordination), results in another 18-VE compound: u(η6-C6Me6)(η4-C6Me6)
*Examples of polyhapto coordinated heterocyclic and inorganic rings: Cr(η5-C4H4S)(CO)3 contains the sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
heterocycle thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
and Cr(η6-B3N3Me6)(CO)3 contains a coordinated inorganic ring (B3N3 ring).

Electrons donated by "π-ligands" versus hapticity
Changes in hapticity
The hapticity of a ligand can change in the course of a reaction. E.g. in a redox reaction: : Here one of the η6-benzene rings changes to a η4-benzene.
Similarly hapticity can change during a substitution reaction:
:
Here one of the η6-benzene rings changes to a η4-benzene.
Similarly hapticity can change during a substitution reaction:
: Here the η5-cyclopentadienyl changes to an η3-cyclopentadienyl, giving room on the metal for an extra 2-electron donating ligand 'L'. Removal of one molecule of CO and again donation of two more electrons by the cyclopentadienyl ligand restores the η5-cyclopentadienyl. The so-called
Here the η5-cyclopentadienyl changes to an η3-cyclopentadienyl, giving room on the metal for an extra 2-electron donating ligand 'L'. Removal of one molecule of CO and again donation of two more electrons by the cyclopentadienyl ligand restores the η5-cyclopentadienyl. The so-called indenyl effect In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related ...
also describes changes in hapticity in a substitution reaction.
Hapticity vs. denticity
Hapticity must be distinguished from denticity. Polydentate ligands coordinate via multiple coordination sites within the ligand. In this case the coordinating atoms are identified using the κ-notation, as for example seen in coordination of1,2-bis(diphenylphosphino)ethane
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (PhPCH) (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.
Preparatio ...
(Ph2PCH2CH2PPh2), to NiCl2 as dichloro thane-1,2-diylbis(diphenylphosphane)-κ2Pickel(II). If the coordinating atoms are contiguous (connected to each other), the η-notation is used, as e.g. in titanocene dichloride: dichlorobis(η5-2,4-cyclopentadien-1-yl)titanium.
Hapticity and fluxionality
Molecules with polyhapto ligands are oftenfluxional
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in s ...
, also known as stereochemically non-rigid. Two classes of fluxionality are prevalent for organometallic complexes of polyhapto ligands:
*Case 1, typically: when the hapticity value is less than the number of sp2 carbon atoms. In such situations, the metal will often migrate from carbon to carbon, maintaining the same net hapticity. The η1-C5H5 ligand in (η5-C5H5)Fe( η1-C5H5)(CO)2 rearranges rapidly in solution such that Fe binds alternatingly to each carbon atom in the η1-C5H5 ligand. This reaction is degenerate and, in the jargon of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, it is an example of a sigmatropic rearrangement. A related example is Bis(cyclooctatetraene)iron, in which the η4- and η6-C8H8 rings interconvert.
*Case 2, typically: complexes containing cyclic polyhapto ligands with maximized hapticity. Such ligands tend to rotate. A famous example is ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
, Fe(η5-C5H5)2, wherein the Cp rings rotate with a low energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
about the principal axis of the molecule that "skewers" each ring (see rotational symmetry
Rotational symmetry, also known as radial symmetry in geometry, is the property a shape has when it looks the same after some rotation by a partial turn. An object's degree of rotational symmetry is the number of distinct orientations in which ...
). This "ring torsion" explains, inter alia, why only one isomer can be isolated for Fe(η5-C5H4Br)2 since the torsional barrier is very low.
References
{{chemical bonds Coordination chemistry