Enriched uranium on:
[Wikipedia]
[Google]
[Amazon]
Enriched uranium is a type of  Enriched uranium is a critical component for both civil nuclear power generation and military
Enriched uranium is a critical component for both civil nuclear power generation and military

 ''Highly enriched uranium'' (HEU) has a 20% or higher concentration of 235U. The fissile uranium in
''Highly enriched uranium'' (HEU) has a 20% or higher concentration of 235U. The fissile uranium in
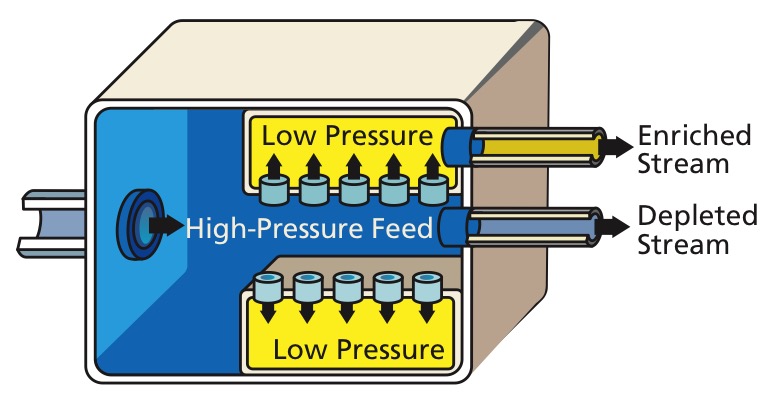 Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (''hex'') through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the
Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (''hex'') through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the
 The gas centrifuge process uses a large number of rotating cylinders in series and parallel formations. Each cylinder's rotation creates a strong
The gas centrifuge process uses a large number of rotating cylinders in series and parallel formations. Each cylinder's rotation creates a strong
 The Zippe-type centrifuge is an improvement on the standard gas centrifuge, the primary difference being the use of heat. The bottom of the rotating cylinder is heated, producing convection currents that move the 235U up the cylinder, where it can be collected by scoops. This improved centrifuge design is used commercially by
The Zippe-type centrifuge is an improvement on the standard gas centrifuge, the primary difference being the use of heat. The bottom of the rotating cylinder is heated, producing convection currents that move the 235U up the cylinder, where it can be collected by scoops. This improved centrifuge design is used commercially by

 Aerodynamic enrichment processes include the Becker jet nozzle techniques developed by E. W. Becker and associates using the
Aerodynamic enrichment processes include the Becker jet nozzle techniques developed by E. W. Becker and associates using the
 In the electromagnetic isotope separation process (EMIS), metallic uranium is first vaporized, and then ionized to positively charged ions. The cations are then accelerated and subsequently deflected by magnetic fields onto their respective collection targets. A production-scale
In the electromagnetic isotope separation process (EMIS), metallic uranium is first vaporized, and then ionized to positively charged ions. The cations are then accelerated and subsequently deflected by magnetic fields onto their respective collection targets. A production-scale
Annotated bibliography on enriched uranium from the Alsos Digital Library for Nuclear Issues
Silex Systems Ltd
, World Nuclear Association
News Resource on Uranium Enrichment
A busy year for SWU (a 2008 review of the commercial enrichment marketplace)
Nuclear Engineering International, 1 September 2008
''Uranium Enrichment and Nuclear Weapon Proliferation'', by Allan S. Krass, Peter Boskma, Boelie Elzen and Wim A. Smit, 296 pp., published for SIPRI by Taylor and Francis Ltd, London, 1983
* * {{DEFAULTSORT:Enriched Uranium Isotope separation Nuclear fuels Uranium, Enriched Nuclear weapon design Uranium
uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
in which the percent composition of uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
(written 235U) has been increased through the process of isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" ...
. Naturally occurring uranium is composed of three major isotopes: uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
(238U with 99.2739–99.2752% natural abundance), uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
(235U, 0.7198–0.7202%), and uranium-234
Uranium-234 (234U or U-234) is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% (55 parts per million) of the raw uranium because its half-life ...
(234U, 0.0050–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be t ...
with thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium wi ...
s.nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bomb ...
s. The International Atomic Energy Agency
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 195 ...
attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation
Nuclear proliferation is the spread of nuclear weapons, fissionable material, and weapons-applicable nuclear technology and information to nations not recognized as " Nuclear Weapon States" by the Treaty on the Non-Proliferation of Nuclear Wea ...
.
There are about 2,000 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s of highly enriched uranium in the world, produced mostly for nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced b ...
, nuclear weapons, naval propulsion, and smaller quantities for research reactor
Research reactors are nuclear fission-based nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or marit ...
s.
The 238U remaining after enrichment is known as depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, hav ...
(DU), and is considerably less radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
than even natural uranium, though still very dense. Depleted uranium is used as a radiation shielding
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposur ...
material and for armor-penetrating weapons.
Grades
Uranium as it is taken directly from the Earth is not suitable as fuel for most nuclear reactors and requires additional processes to make it usable (CANDU
The CANDU (Canada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide ( heavy water) moderator and its use of (originally, natural) uranium fuel. C ...
design is a notable exception). Uranium is mined either underground or in an open pit depending on the depth at which it is found. After the uranium ore is mined, it must go through a milling process to extract the uranium from the ore.
This is accomplished by a combination of chemical processes with the end product being concentrated uranium oxide, which is known as " yellowcake", contains roughly 80% uranium whereas the original ore typically contains as little as 0.1% uranium.
After the milling process is complete, the uranium must next undergo a process of conversion, "to either uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear re ...
, which can be used as the fuel for those types of reactors that do not require enriched uranium, or into uranium hexafluoride, which can be enriched to produce fuel for the majority of types of reactors". Naturally-occurring uranium is made of a mixture of 235U and 238U. The 235U is fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be t ...
, meaning it is easily split with neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s while the remainder is 238U, but in nature, more than 99% of the extracted ore is 238U. Most nuclear reactors require enriched uranium, which is uranium with higher concentrations of 235U ranging between 3.5% and 4.5% (although a few reactor designs using a graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
or heavy water moderator, such as the RBMK and CANDU
The CANDU (Canada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide ( heavy water) moderator and its use of (originally, natural) uranium fuel. C ...
, are capable of operating with natural uranium as fuel). There are two commercial enrichment processes: gaseous diffusion and gas centrifugation
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radiu ...
. Both enrichment processes involve the use of uranium hexafluoride and produce enriched uranium oxide.

Reprocessed uranium (RepU)
''Reprocessed uranium'' (RepU) is a product ofnuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in w ...
s involving nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, th ...
of spent fuel. RepU recovered from light water reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron react ...
(LWR) spent fuel typically contains slightly more 235U than natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes ...
, and therefore could be used to fuel reactors that customarily use natural uranium as fuel, such as CANDU reactors. It also contains the undesirable isotope uranium-236, which undergoes neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
, wasting neutrons (and requiring higher 235U enrichment) and creating neptunium-237
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be sy ...
, which would be one of the more mobile and troublesome radionuclides in deep geological repository
A deep geological repository is a way of storing hazardous or radioactive waste within a stable geologic environment (typically 200–1000 m deep). It entails a combination of waste form, waste package, engineered seals and geology that is suite ...
disposal of nuclear waste.
Low-enriched uranium (LEU)
''Low-enriched uranium'' (LEU) has a lower than 20% concentration of 235U; for instance, in commercial LWR, the most prevalent power reactors in the world, uranium is enriched to 3 to 5% 235U. Slightly enriched uranium (SEU) has a concentration of under 2% 235U. High-assay LEU (HALEU) is enriched from 5–20%. Fresh LEU used inresearch reactor
Research reactors are nuclear fission-based nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or marit ...
s is usually enriched 12 to 19.75% 235U, the latter concentration is used to replace HEU fuels when converting to LEU.
Highly enriched uranium (HEU)
 ''Highly enriched uranium'' (HEU) has a 20% or higher concentration of 235U. The fissile uranium in
''Highly enriched uranium'' (HEU) has a 20% or higher concentration of 235U. The fissile uranium in nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bomb ...
primaries usually contains 85% or more of 235U known as weapons-grade, though theoretically for an implosion design, a minimum of 20% could be sufficient (called weapon-usable) although it would require hundreds of kilograms of material and "would not be practical to design"; even lower enrichment is hypothetically possible, but as the enrichment percentage decreases the critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
for unmoderated fast neutrons rapidly increases, with for example, an infinite mass of 5.4% 235U being required. For criticality experiments, enrichment of uranium to over 97% has been accomplished.
The very first uranium bomb, Little Boy
"Little Boy" was the type of atomic bomb dropped on the Japanese city of Hiroshima on 6 August 1945 during World War II, making it the first nuclear weapon used in warfare. The bomb was dropped by the Boeing B-29 Superfortress ''Enola Gay'' p ...
, dropped by the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
on Hiroshima
is the capital of Hiroshima Prefecture in Japan. , the city had an estimated population of 1,199,391. The gross domestic product (GDP) in Greater Hiroshima, Hiroshima Urban Employment Area, was US$61.3 billion as of 2010. Kazumi Matsui ...
in 1945, used 64 kilograms of 80% enriched uranium. Wrapping the weapon's fissile core in a neutron reflector (which is standard on all nuclear explosives) can dramatically reduce the critical mass. Because the core was surrounded by a good neutron reflector, at explosion it comprised almost 2.5 critical masses. Neutron reflectors, compressing the fissile core via implosion, fusion boosting, and "tamping", which slows the expansion of the fissioning core with inertia, allow nuclear weapon design
Nuclear weapon designs are physical, chemical, and engineering arrangements that cause the physics package of a nuclear weapon to detonate. There are three existing basic design types:
* pure fission weapons, the simplest and least technically ...
s that use less than what would be one bare-sphere critical mass at normal density. The presence of too much of the 238U isotope inhibits the runaway nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
that is responsible for the weapon's power. The critical mass for 85% highly enriched uranium is about , which at normal density would be a sphere about in diameter.
Later US nuclear weapons usually use plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
in the primary stage, but the jacket or tamper secondary stage, which is compressed by the primary nuclear explosion often uses HEU with enrichment between 40% and 80%
along with the fusion fuel lithium deuteride
Lithium hydride is an inorganic compound with the formula Li H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not solub ...
. For the secondary of a large nuclear weapon, the higher critical mass of less-enriched uranium can be an advantage as it allows the core at explosion time to contain a larger amount of fuel. The 238U is not said to be fissile but still is fissionable by fast neutrons (>2 MeV) such as the ones produced during D-T fusion.
HEU is also used in fast neutron reactors, whose cores require about 20% or more of fissile material, as well as in naval reactors
Naval Reactors (NR), also known as the Naval Nuclear Propulsion Program, is an umbrella term for the U.S. government office that has comprehensive responsibility for the safe and reliable operation of the United States Navy's nuclear propulsion p ...
, where it often contains at least 50% 235U, but typically does not exceed 90%. The Fermi-1 commercial fast reactor prototype used HEU with 26.5% 235U. Significant quantities of HEU are used in the production of medical isotopes, for example molybdenum-99
Molybdenum (42Mo) has 33 known isotopes, ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum ...
for technetium-99m generators.
As of 2022, Russia
Russia (, , ), or the Russian Federation, is a transcontinental country spanning Eastern Europe and Northern Asia. It is the largest country in the world, with its internationally recognised territory covering , and encompassing one-ei ...
was the only country that sells HALEU fuel that. Next-generation nuclear reactor developers such as TerraPower rely on Russian supplies. Boycotts created in response to Russia's invasion of Ukraine have interrupted shipments of this material.
Enrichment methods
Isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" ...
is difficult because two isotopes of the same element have nearly identical chemical properties, and can only be separated gradually using small mass differences. (235U is only 1.26% lighter than 238U). This problem is compounded because uranium is rarely separated in its atomic form, but instead as a compound (235UF6 is only 0.852% lighter than 238UF6).
A cascade
Cascade, Cascades or Cascading may refer to:
Science and technology Science
*Cascade waterfalls, or series of waterfalls
* Cascade, the CRISPR-associated complex for antiviral defense (a protein complex)
* Cascade (grape), a type of fruit
* Bioc ...
of identical stages produces successively higher concentrations of 235U. Each stage passes a slightly more concentrated product to the next stage and returns a slightly less concentrated residue to the previous stage.
There are currently two generic commercial methods employed internationally for enrichment: gaseous diffusion (referred to as ''first'' generation) and gas centrifuge
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radiu ...
(''second'' generation), which consumes only 2% to 2.5% as much energy as gaseous diffusion (at least a "factor of 20" more efficient). Some work is being done that would use nuclear resonance; however there is no reliable evidence that any nuclear resonance processes have been scaled up to production.
Diffusion techniques
Gaseous diffusion
 Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (''hex'') through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the
Gaseous diffusion is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (''hex'') through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because t ...
, gaseous diffusion played a major role as a uranium enrichment technique, and as of 2008 accounted for about 33% of enriched uranium production, but in 2011 was deemed an obsolete technology that is steadily being replaced by the later generations of technology as the diffusion plants reach their ends-of-life. In 2013, the Paducah facility in the US ceased operating, it was the last commercial 235U gaseous diffusion plant in the world.
Thermal diffusion
Thermal diffusion uses the transfer of heat across a thin liquid or gas to accomplish isotope separation. The process exploits the fact that the lighter 235U gas molecules will diffuse toward a hot surface, and the heavier 238U gas molecules will diffuse toward a cold surface. The S-50 plant atOak Ridge, Tennessee
Oak Ridge is a city in Anderson County, Tennessee, Anderson and Roane County, Tennessee, Roane counties in the East Tennessee, eastern part of the U.S. state of Tennessee, about west of downtown Knoxville, Tennessee, Knoxville. Oak Ridge's popu ...
was used during World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the World War II by country, vast majority of the world's countries—including all of the great power ...
to prepare feed material for the EMIS process. It was abandoned in favor of gaseous diffusion.
Centrifuge techniques
Gas centrifuge
 The gas centrifuge process uses a large number of rotating cylinders in series and parallel formations. Each cylinder's rotation creates a strong
The gas centrifuge process uses a large number of rotating cylinders in series and parallel formations. Each cylinder's rotation creates a strong centripetal force
A centripetal force (from Latin ''centrum'', "center" and ''petere'', "to seek") is a force that makes a body follow a curved path. Its direction is always orthogonal to the motion of the body and towards the fixed point of the instantaneous c ...
so that the heavier gas molecules containing 238U move tangentially toward the outside of the cylinder and the lighter gas molecules rich in 235U collect closer to the center. It requires much less energy to achieve the same separation than the older gaseous diffusion process, which it has largely replaced and so is the current method of choice and is termed ''second generation''. It has a separation factor per stage of 1.3 relative to gaseous diffusion of 1.005, which translates to about one-fiftieth of the energy requirements. Gas centrifuge techniques produce close to 100% of the world's enriched uranium. The cost per separative work unit is approximately 100 dollars per SWU, making it about 40% cheaper than standard gaseous diffusion techniques.
Zippe centrifuge
Urenco
The Urenco Group is a British-German-Dutch nuclear fuel consortium operating several uranium enrichment plants in Germany, the Netherlands, United States, and United Kingdom. It supplies nuclear power stations in about 15 countries, and stat ...
to produce nuclear fuel and was used by Pakistan
Pakistan ( ur, ), officially the Islamic Republic of Pakistan ( ur, , label=none), is a country in South Asia. It is the world's List of countries and dependencies by population, fifth-most populous country, with a population of almost 24 ...
in their nuclear weapons program.
Laser techniques
Laser processes promise lower energy inputs, lower capital costs and lower tails assays, hence significant economic advantages. Several laser processes have been investigated or are under development. ''Separation of isotopes by laser excitation'' (SILEX
Silex is any of various forms of ground stone. In modern contexts the word refers to a finely ground, nearly pure form of silica or silicate.
In the late 16th century, it meant powdered or ground up " flints" (i.e. stones, generally meaning the ...
) is well developed and is licensed for commercial operation as of 2012. Separation of isotopes by laser excitation is a very effective and cheap method of uranium separation, able to be done in small facilities requiring much less energy and space than previous separation techniques. The cost of uranium enrichment using laser enrichment technologies is approximately $30 per SWU which is less than a third of the price of gas centrifuges, the current standard of enrichment. Separation of isotopes by laser excitation could be done in facilities virtually undetectable by satellites. More than 20 countries have worked with laser separation over the past two decades, the most notable of these countries being Iran and North Korea, though all countries have had very limited success up to this point.
Atomic vapor laser isotope separation (AVLIS)
'' Atomic vapor laser isotope separation'' employs specially tuned lasers to separate isotopes of uranium using selective ionization ofhyperfine transitions
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucl ...
. The technique uses laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The ...
s tuned to frequencies that ionize 235U atoms and no others. The positively charged 235U ions are then attracted to a negatively charged plate and collected.
Molecular laser isotope separation (MLIS)
'' Molecular laser isotope separation'' uses an infrared laser directed at UF6, exciting molecules that contain a 235U atom. A second laser frees afluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
atom, leaving uranium pentafluoride, which then precipitates out of the gas.
Separation of isotopes by laser excitation (SILEX)
'' Separation of isotopes by laser excitation'' is an Australian development that also uses UF6. After a protracted development process involving U.S. enrichment company USEC acquiring and then relinquishing commercialization rights to the technology, GE Hitachi Nuclear Energy (GEH) signed a commercialization agreement with Silex Systems in 2006. GEH has since built a demonstration test loop and announced plans to build an initial commercial facility. Details of the process are classified and restricted by intergovernmental agreements between United States, Australia, and the commercial entities. SILEX has been projected to be an order of magnitude more efficient than existing production techniques but again, the exact figure is classified. In August, 2011 Global Laser Enrichment, a subsidiary of GEH, applied to the U.S.Nuclear Regulatory Commission
The Nuclear Regulatory Commission (NRC) is an independent agency of the United States government tasked with protecting public health and safety related to nuclear energy. Established by the Energy Reorganization Act of 1974, the NRC began opera ...
(NRC) for a permit to build a commercial plant. In September 2012, the NRC issued a license for GEH to build and operate a commercial SILEX enrichment plant, although the company had not yet decided whether the project would be profitable enough to begin construction, and despite concerns that the technology could contribute to nuclear proliferation
Nuclear proliferation is the spread of nuclear weapons, fissionable material, and weapons-applicable nuclear technology and information to nations not recognized as " Nuclear Weapon States" by the Treaty on the Non-Proliferation of Nuclear Wea ...
. The fear of nuclear proliferation arose in part due to laser separation technology requiring less than 25% of the space of typical separation techniques, as well as only requiring the amount of energy that would power 12 typical houses, putting a laser separation plant that works by means of laser excitation well below the detection threshold of existing surveillance technologies. Due to these concerns the American Physical Society
The American Physical Society (APS) is a not-for-profit membership organization of professionals in physics and related disciplines, comprising nearly fifty divisions, sections, and other units. Its mission is the advancement and diffusion of k ...
filed a petition with the U.S. Nuclear Regulatory Commission
The Nuclear Regulatory Commission (NRC) is an independent agency of the United States government tasked with protecting public health and safety related to nuclear energy. Established by the Energy Reorganization Act of 1974, the NRC began operat ...
, asking that before any laser excitation plants are built that they undergo a formal review of proliferation risks. The APS even went as far as calling the technology a "game changer" due to the ability for it to be hidden from any type of detection.
Other techniques
Aerodynamic processes
 Aerodynamic enrichment processes include the Becker jet nozzle techniques developed by E. W. Becker and associates using the
Aerodynamic enrichment processes include the Becker jet nozzle techniques developed by E. W. Becker and associates using the LIGA
Liga or LIGA may refer to:
People
* Līga (name), a Latvian female given name
* Luciano Ligabue, more commonly known as Ligabue or ''Liga'', Italian rock singer-songwriter
Sports
* Liga ACB, men's professional basketball league in Spain
* Lig ...
process and the vortex tube
The vortex tube, also known as the Ranque-Hilsch vortex tube, is a mechanical device that separates a compressed gas into hot and cold streams. The gas emerging from the hot end can reach temperatures of , and the gas emerging from the cold end ...
separation process. These aerodynamic
Aerodynamics, from grc, ἀήρ ''aero'' (air) + grc, δυναμική (dynamics), is the study of the motion of air, particularly when affected by a solid object, such as an airplane wing. It involves topics covered in the field of fluid dyn ...
separation processes depend upon diffusion driven by pressure gradients, as does the gas centrifuge. They in general have the disadvantage of requiring complex systems of cascading of individual separating elements to minimize energy consumption. In effect, aerodynamic processes can be considered as non-rotating centrifuges. Enhancement of the centrifugal forces is achieved by dilution of UF6 with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
or helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
as a carrier gas achieving a much higher flow velocity for the gas than could be obtained using pure uranium hexafluoride. The Uranium Enrichment Corporation of South Africa (UCOR) developed and deployed the continuous Helikon vortex separation cascade for high production rate low-enrichment and the substantially different semi-batch Pelsakon low production rate high enrichment cascade both using a particular vortex tube separator design, and both embodied in industrial plant. A demonstration plant was built in Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
by NUCLEI, a consortium led by Industrias Nucleares do Brasil that used the separation nozzle process. However all methods have high energy consumption and substantial requirements for removal of waste heat; none is currently still in use.
Electromagnetic isotope separation
mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
named the Calutron
A calutron is a mass spectrometer originally designed and used for separating the isotopes of uranium. It was developed by Ernest Lawrence during the Manhattan Project and was based on his earlier invention, the cyclotron. Its name was deri ...
was developed during World War II that provided some of the 235U used for the Little Boy
"Little Boy" was the type of atomic bomb dropped on the Japanese city of Hiroshima on 6 August 1945 during World War II, making it the first nuclear weapon used in warfare. The bomb was dropped by the Boeing B-29 Superfortress ''Enola Gay'' p ...
nuclear bomb, which was dropped over Hiroshima
is the capital of Hiroshima Prefecture in Japan. , the city had an estimated population of 1,199,391. The gross domestic product (GDP) in Greater Hiroshima, Hiroshima Urban Employment Area, was US$61.3 billion as of 2010. Kazumi Matsui ...
in 1945. Properly the term 'Calutron' applies to a multistage device arranged in a large oval around a powerful electromagnet. Electromagnetic isotope separation has been largely abandoned in favour of more effective methods.
Chemical methods
One chemical process has been demonstrated to pilot plant stage but not used for production. The French CHEMEX process exploited a very slight difference in the two isotopes' propensity to change valency in oxidation/reduction, using immiscible aqueous and organic phases. An ion-exchange process was developed by the Asahi Chemical Company inJapan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
that applies similar chemistry but effects separation on a proprietary resin ion-exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
column.
Plasma separation
Plasma separation process (PSP) describes a technique that makes use ofsuperconducting magnet
A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire has no electrical resistance and therefore can conduct much ...
s and . In this process, the principle of ion cyclotron resonance is used to selectively energize the 235U isotope in a plasma containing a mix of ions. France developed its own version of PSP, which it called RCI. Funding for RCI was drastically reduced in 1986, and the program was suspended around 1990, although RCI is still used for stable isotope separation.
Separative work unit
"Separative work" – the amount of separation done by an enrichment process – is a function of the concentrations of the feedstock, the enriched output, and the depleted tailings; and is expressed in units that are so calculated as to be proportional to the total input (energy / machine operation time) and to the mass processed. Separative work is ''not'' energy. The same amount of separative work will require different amounts of energy depending on the efficiency of the separation technology. Separative work is measured in ''Separative work units'' SWU, kg SW, or kg UTA (from the German ''Urantrennarbeit'' – literally ''uranium separation work'') * 1 SWU = 1 kg SW = 1 kg UTA * 1 kSWU = 1 tSW = 1 t UTA * 1 MSWU = 1 ktSW = 1 kt UTACost issues
In addition to the separative work units provided by an enrichment facility, the other important parameter to be considered is the mass of natural uranium (NU) that is needed to yield a desired mass of enriched uranium. As with the number of SWUs, the amount of feed material required will also depend on the level of enrichment desired and upon the amount of 235U that ends up in the depleted uranium. However, unlike the number of SWUs required during enrichment, which increases with decreasing levels of 235U in the depleted stream, the amount of NU needed will decrease with decreasing levels of 235U that end up in the DU. For example, in the enrichment of LEU for use in a light water reactor it is typical for the enriched stream to contain 3.6% 235U (as compared to 0.7% in NU) while the depleted stream contains 0.2% to 0.3% 235U. In order to produce one kilogram of this LEU it would require approximately 8 kilograms of NU and 4.5 SWU if the DU stream was allowed to have 0.3% 235U. On the other hand, if the depleted stream had only 0.2% 235U, then it would require just 6.7 kilograms of NU, but nearly 5.7 SWU of enrichment. Because the amount of NU required and the number of SWUs required during enrichment change in opposite directions, if NU is cheap and enrichment services are more expensive, then the operators will typically choose to allow more 235U to be left in the DU stream whereas if NU is more expensive and enrichment is less so, then they would choose the opposite. When converting uranium (hexafluoride, hex for short) to metal, 0.3% is lost during manufacturing.Downblending
The opposite of enriching is downblending; surplus HEU can be downblended to LEU to make it suitable for use in commercial nuclear fuel. The HEU feedstock can contain unwanted uranium isotopes: 234U is a minor isotope contained in natural uranium (primarily as a product ofalpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of - because the half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of is much larger than that of , it'll be produced and destroyed at the same rate in a constant steady state equilibrium, bringing any sample with sufficient content to a stable ratio of to over long enough timescales); during the enrichment process, its concentration increases but remains well below 1%. High concentrations of 236U are a byproduct from irradiation in a reactor and may be contained in the HEU, depending on its manufacturing history. is produced primarily when absorbs a neutron and does not fission. The production of is thus unavoidable in any thermal neutron reactor with fuel. HEU reprocessed from nuclear weapons material production reactors (with an 235U assay of approx. 50%) may contain 236U concentrations as high as 25%, resulting in concentrations of approximately 1.5% in the blended LEU product. 236U is a neutron poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable eff ...
; therefore the actual 235U concentration in the LEU product must be raised accordingly to compensate for the presence of 236U. While also absorbs neutrons, it is a fertile material
Fertile material is a material that, although not itself fissionable by thermal neutrons, can be converted into a fissile material by neutron absorption and subsequent nuclei conversions.
Naturally occurring fertile materials
Naturally occurring ...
that is turned into fissile upon neutron absorption. If absorbs a neutron, the resulting short-lived beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s to , which is not usable in thermal neutron reactors but can be chemically separated from spent fuel to be disposed of as waste or to be transmutated into (for use in nuclear batteries) in special reactors.
The blendstock can be NU, or DU, however depending on feedstock quality, SEU at typically 1.5 wt% 235U may be used as a blendstock to dilute the unwanted byproducts that may be contained in the HEU feed. Concentrations of these isotopes in the LEU product in some cases could exceed ASTM
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, an ...
specifications for nuclear fuel, if NU, or DU were used. So, the HEU downblending generally cannot contribute to the waste management problem posed by the existing large stockpiles of depleted uranium. At present, 95 percent of the world's stocks of depleted uranium remain in secure storage.
A major downblending undertaking called the Megatons to Megawatts Program The Megatons to Megawatts Program, also called the United States-Russia Highly Enriched Uranium Purchase Agreement, was an agreement between Russia and the United States. The official name of the program is the "Agreement between the Government of t ...
converts ex-Soviet weapons-grade HEU to fuel for U.S. commercial power reactors. From 1995 through mid-2005, 250 tonnes of high-enriched uranium (enough for 10,000 warheads) was recycled into low-enriched-uranium. The goal is to recycle 500 tonnes by 2013. The decommissioning programme of Russian nuclear warheads accounted for about 13% of total world requirement for enriched uranium leading up to 2008.
The United States Enrichment Corporation has been involved in the disposition of a portion of the 174.3 tonnes of highly enriched uranium (HEU) that the U.S. government declared as surplus military material in 1996. Through the U.S. HEU Downblending Program, this HEU material, taken primarily from dismantled U.S. nuclear warheads, was recycled into low-enriched uranium (LEU) fuel, used by nuclear power plants
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a generator that produces ...
to generate electricity.
Global enrichment facilities
The following countries are known to operate enrichment facilities: Argentina, Brazil, China, France, Germany, India, Iran, Japan, the Netherlands, North Korea, Pakistan, Russia, the United Kingdom, and the United States. Belgium, Iran, Italy, and Spain hold an investment interest in the FrenchEurodif
Eurodif, which means ''European Gaseous Diffusion Uranium Enrichment Consortium'', is a subsidiary of the French company Orano, which operates a uranium enrichment plant established at the Tricastin Nuclear Power Center in Pierrelatte in Drôm ...
enrichment plant, with Iran's holding entitling it to 10% of the enriched uranium output. Countries that had enrichment programs in the past include Libya and South Africa, although Libya's facility was never operational. The Australian company Silex Systems has developed a laser enrichment process known as SILEX
Silex is any of various forms of ground stone. In modern contexts the word refers to a finely ground, nearly pure form of silica or silicate.
In the late 16th century, it meant powdered or ground up " flints" (i.e. stones, generally meaning the ...
(separation of isotopes by laser excitation), which it intends to pursue through financial investment in a U.S. commercial venture by General Electric, Although SILEX has been granted a license to build a plant, the development is still in its early stages as laser enrichment has yet to be proven to be economically viable, and there is a petition being filed to review the license given to SILEX over nuclear proliferation concerns. It has also been claimed that Israel has a uranium enrichment program housed at the Negev Nuclear Research Center
The Shimon Peres Negev Nuclear Research Center ( he, קריה למחקר גרעיני – נגב ע"ש שמעון פרס, formerly the ''Negev Nuclear Research Center'', unofficially sometimes referred to as the ''Dimona reactor'') is an Israe ...
site near Dimona.
Codename
During theManhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, weapons-grade highly enriched uranium was given the codename oralloy, a shortened version of Oak Ridge alloy, after the location of the plants where the uranium was enriched. The term ''oralloy'' is still occasionally used to refer to enriched uranium.
See also
*List of laser articles
This is a list of laser topics.
A
* 3D printing, additive manufacturing
* Abnormal reflection
* Above-threshold ionization
* Absorption spectroscopy
* Accelerator physics
* Acoustic microscopy
* Acousto-optic deflector
* Acousto-optic ...
* MOX fuel
* Nuclear fuel bank
* Orano
* Uranium market
* Uranium mining
Uranium mining is the process of extraction of uranium ore from the ground. Over 50 thousand tons of uranium were produced in 2019. Kazakhstan, Canada, and Australia were the top three uranium producers, respectively, and together account f ...
References
External links
Annotated bibliography on enriched uranium from the Alsos Digital Library for Nuclear Issues
Silex Systems Ltd
, World Nuclear Association
News Resource on Uranium Enrichment
A busy year for SWU (a 2008 review of the commercial enrichment marketplace)
Nuclear Engineering International, 1 September 2008
''Uranium Enrichment and Nuclear Weapon Proliferation'', by Allan S. Krass, Peter Boskma, Boelie Elzen and Wim A. Smit, 296 pp., published for SIPRI by Taylor and Francis Ltd, London, 1983
* * {{DEFAULTSORT:Enriched Uranium Isotope separation Nuclear fuels Uranium, Enriched Nuclear weapon design Uranium