|
Nuclear Weapon Design
Nuclear weapon designs are physical, chemical, and engineering arrangements that cause the physics package of a nuclear weapon to detonate. There are three existing basic design types: * pure fission weapons, the simplest and least technically demanding, were the first nuclear weapons built and have so far been the only type ever used in warfare (by the United States on Japan during WWII). * boosted fission weapons increase yield beyond that of the implosion design by using small quantities of fusion fuel to enhance the fission chain reaction. Boosting can more than double the weapon's fission energy yield. * staged thermonuclear weapons are essentially arrangements of two or more "stages", most usually two. The first stage is normally a boosted fission weapon as above (except for the earliest thermonuclear weapons, which used a pure fission weapon instead). Its detonation causes it to shine intensely with x-radiation, which illuminates and implodes the second stage filled wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Gadget In The Trinity Test Site Tower (1945)
''The'' () is a grammatical article in English, denoting persons or things already mentioned, under discussion, implied or otherwise presumed familiar to listeners, readers, or speakers. It is the definite article in English. ''The'' is the most frequently used word in the English language; studies and analyses of texts have found it to account for seven percent of all printed English-language words. It is derived from gendered articles in Old English which combined in Middle English and now has a single form used with pronouns of any gender. The word can be used with both singular and plural nouns, and with a noun that starts with any letter. This is different from many other languages, which have different forms of the definite article for different genders or numbers. Pronunciation In most dialects, "the" is pronounced as (with the voiced dental fricative followed by a schwa) when followed by a consonant sound, and as (homophone of pronoun '' thee'') when followed by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
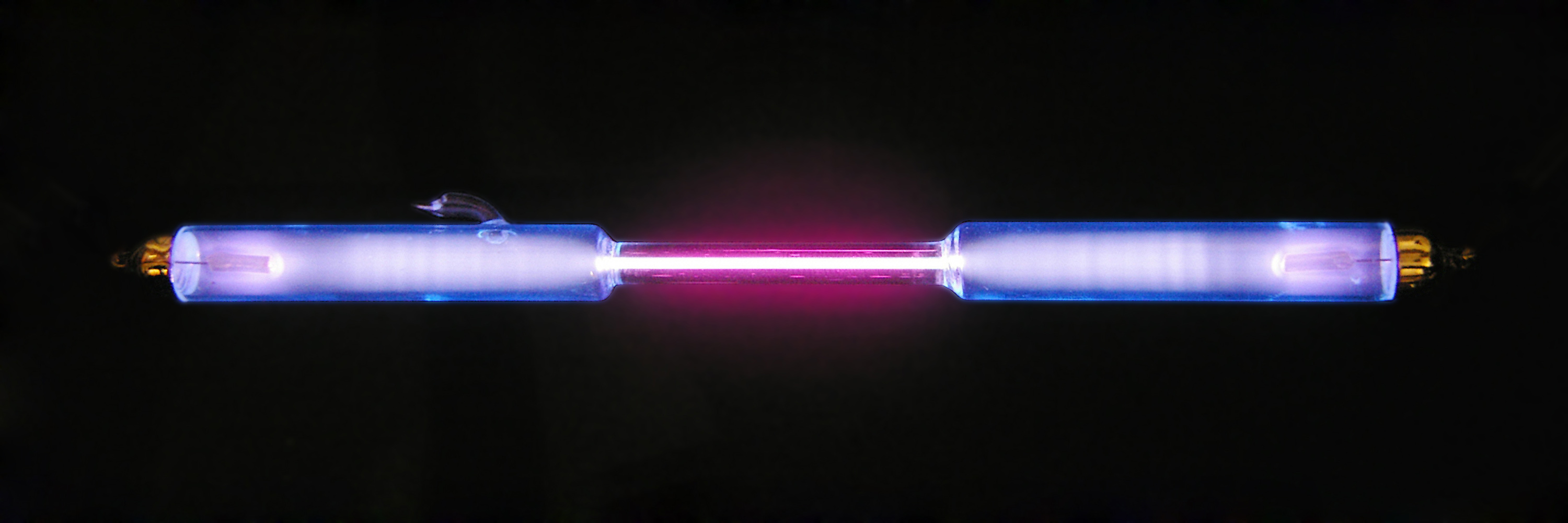
Coulomb Collision
A Coulomb collision is a binary elastic collision between two charged particles interacting through their own electric field. As with any inverse-square law, the resulting trajectories of the colliding particles is a hyperbolic Keplerian orbit. This type of collision is common in plasmas where the typical kinetic energy of the particles is too large to produce a significant deviation from the initial trajectories of the colliding particles, and the cumulative effect of many collisions is considered instead. Simplified mathematical treatment for plasmas In a plasma, a Coulomb collision rarely results in a large deflection. The cumulative effect of the many small angle collisions, however, is often larger than the effect of the few large angle collisions that occur, so it is instructive to consider the collision dynamics in the limit of small deflections. We can consider an electron of charge -e and mass m_e passing a stationary ion of charge +Ze and much larger mass at a distance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements. It is the second lightest and second most abundant element in the observable universe ( hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, the vast majority of which was forme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Lithium
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for lithium-6 and for lithium-7) when compared with the adjacent lighter and heavier elements, helium ( for helium-4) and beryllium ( for beryllium-9). The longest-lived radioisotope of lithium is lithium-8, which has a half-life of just . Lithium-9 has a half-life of , and lithium-11 has a half-life of . All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is lithium-4, which decays by proton emission with a half-life of about (), although the half-life of lithium-3 is yet to be determined, and is likely to be much shorter, like helium-2 (diproton) which undergoes proton emission within s. Lithium-7 and lithium-6 are two of the primordial nuclides that were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). ( Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denote the two particles composing the nucleus. De ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Highly Enriched Uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2739–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7202%), and uranium-234 (234U, 0.0050–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation. There are about 2,000 tonnes of highly enriched uranium in the world, produced mostly for nuclear power, nuclear weapons, naval propulsion, and smaller quantities for research reactors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typified by either slow neutrons (i.e., a thermal system) or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives. Fissile vs fissionable According to the Ronen fissile rule, for a heavy element with 90 ≤ ''Z'' ≤ 100, its isotopes with , with few exceptions, are fissile (where ''N'' = number of neutrons and ''Z'' = number of protons).The fissile rule thus formulated indicates 33 isotopes as likely fissile: Th-225, 227, 229; Pa-228, 230, 232; U-231, 233, 235; Np-234, 236, 238; Pu-237, 239, 241; Am-240, 242, 244; Cm-243, 245, 247; Bk-246, 248, 250; Cf-249, 251, 253; Es-252, 254, 256; Fm-255, 257, 259. Only fourteen (including a long ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Black-body Radiation
Black-body radiation is the thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific, continuous spectrum of wavelengths, inversely related to intensity, that depend only on the body's temperature, which is assumed, for the sake of calculations and theory, to be uniform and constant., Chapter 13. A perfectly insulated enclosure which is in thermal equilibrium internally contains black-body radiation, and will emit it through a hole made in its wall, provided the hole is small enough to have a negligible effect upon the equilibrium. The thermal radiation spontaneously emitted by many ordinary objects can be approximated as black-body radiation. Of particular importance, although planets and stars (including the Earth and Sun) are neither in thermal equilibrium with their surroundings nor perfect black bodies, black-body radiation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
.png)