|
Rubidium Oxide
Rubidium oxide is the chemical compound with the formula . Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of . In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. A major source of rubidium is lepidolite, , wherein Rb sometimes replaces K. is a yellow colored solid. The related species , and are colorless, pale-yellow, and orange, respectively. The alkali metal oxides crystallise in the antifluorite structure. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 4-coordinate (tetrahedral) and oxide ions 8-coordinate (cubic). Properties Like other alkali metal oxides, Rb2O is a strong base. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. :Rb2O + H2O → 2 RbOH So re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Hydroxide
Rubidium hydroxide is the inorganic compound with the formula RbOH. It consists of rubidium cations and an equal number of hydroxide anions. It is a colorless solid that is commercially available as aqueous solutions from a few suppliers. Like other strong bases, rubidium hydroxide is highly corrosive. Rubidium hydroxide is formed when rubidium metal reacts with water. Uses Rubidium hydroxide is rarely used in industrial processes because potassium hydroxide and sodium hydroxide can perform nearly all the same functions of rubidium hydroxide. Metal oxide catalysts are sometimes modified with rubidium hydroxide. See also *Potassium hydroxide *Sodium hydroxide *Rubidium Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ... References * * {{DEFAULTSORT:Rubidium Hydroxide Rub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium Hydroxide
Rubidium hydroxide is the inorganic compound with the formula RbOH. It consists of rubidium cations and an equal number of hydroxide anions. It is a colorless solid that is commercially available as aqueous solutions from a few suppliers. Like other strong bases, rubidium hydroxide is highly corrosive. Rubidium hydroxide is formed when rubidium metal reacts with water. Uses Rubidium hydroxide is rarely used in industrial processes because potassium hydroxide and sodium hydroxide can perform nearly all the same functions of rubidium hydroxide. Metal oxide catalysts are sometimes modified with rubidium hydroxide. See also *Potassium hydroxide *Sodium hydroxide *Rubidium Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ... References * * {{DEFAULTSORT:Rubidium Hydroxide Rub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities. Chemical structure The compound crystallizes in a cubic motif called the fluorite structure. Ca2+ centres are eight-coordinate, being centered in a cube of eight F− centres. Each F− centre is coordinated to four Ca2+ centres in the shape of a tetrahedron. Although perfectly packed crystalline samples are colorless, the mineral is often deeply colored due to the presence of F-centers. The same crystal structure is found in numerous ionic compounds with formula AB2, such as CeO2, cubic ZrO2, UO2, ThO2, and PuO2. In the corresponding anti-structure, called the antifluorite structure, anions and cations are swapped, such as Be2C. Gas phase The gas phase is noteworthy for failing the predictions of VSEPR theory; the molecule is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal Oxide
The alkali metals react with oxygen to form several different compounds: suboxides, oxides, peroxides, sesquioxides, superoxides, and ozonides. They all react violently with water. Alkali metal suboxides * Hexarubidium monoxide (Rb6O) h * Nonarubidium dioxide (Rb9O2) * Tricaesium monoxide (Cs3O) is a dark green solid. * Tetracaesium monoxide (Cs4O) * Heptacaesium monoxide (Cs7O) * Tricaesium dioxide (Cs3O2) * Heptacaesium dioxide (Cs7O2) * Undecacaesium trioxide (Cs11O3) * Undecacaesium monorubidium trioxide (Cs11RbO3) * Undecacaesium dirubidium trioxide (Cs11Rb2O3) * Undecacaesium trirubidium trioxide (Cs11Rb3O3) Alkali metal oxides * Lithium oxide (Li2O) is the lightest alkali metal oxide and a white solid. It melts at 1570 °C. *Sodium oxide (Na2O) is a white solid that melts at 1132 °C and decomposes at 1950 °C. It is a component of glass. *Potassium oxide (K2O) is a pale yellow solid that decomposes at 350 °C. * Rubidium oxide (Rb2O) is a yellow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lepidolite
Lepidolite is a lilac-gray or rose-colored member of the mica group of minerals with chemical formula . It is the most abundant lithium-bearing mineral and is a secondary source of this metal. It is the major source of the alkali metal rubidium. Lepidolite is found with other lithium-bearing minerals, such as spodumene, in pegmatite bodies. It has also been found in high-temperature quartz veins, greisens and granite. Description Lepidolite is a phyllosilicate mineral and a member of the polylithionite-trilithionite series. Lepidolite is part of a three-part series consisting of polylithionite, lepidolite, and trilithionite. All three minerals share similar properties and are caused because of varying ratios of lithium and aluminum in their chemical formulas. The Li:Al ratio varies from 2:1 in polylithionite up to 1.5:1.5 in trilithionite. Lepidolite is found naturally in a variety of colors, mainly pink, purple, and red, but also gray and, rarely, yellow and colorless. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminosilicate
Aluminosilicate minerals ( IMA symbol: Als) are minerals composed of aluminium, silicon, and oxygen, plus countercations. They are a major component of kaolin and other clay minerals. Andalusite, kyanite, and sillimanite are naturally occurring aluminosilicate minerals that have the composition Al2 Si O5. The triple point of the three polymorphs is located at a temperature of and a pressure of . These three minerals are commonly used as index minerals in metamorphic rocks. Naturally occurring microporous, hydrous aluminosilicate minerals are referred to as zeolites. Feldspar is a common tectosilicate aluminosilicate mineral made of potassium, sodium, and calcium cations surrounded by a negatively charged network of silicon, aluminium and oxygen atoms. The catalyst silica-alumina is an amorphous substance which is not an aluminosilicate compound. Aluminosilicate glasses There exist a wide variety of glass types. The characteristics of these different ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
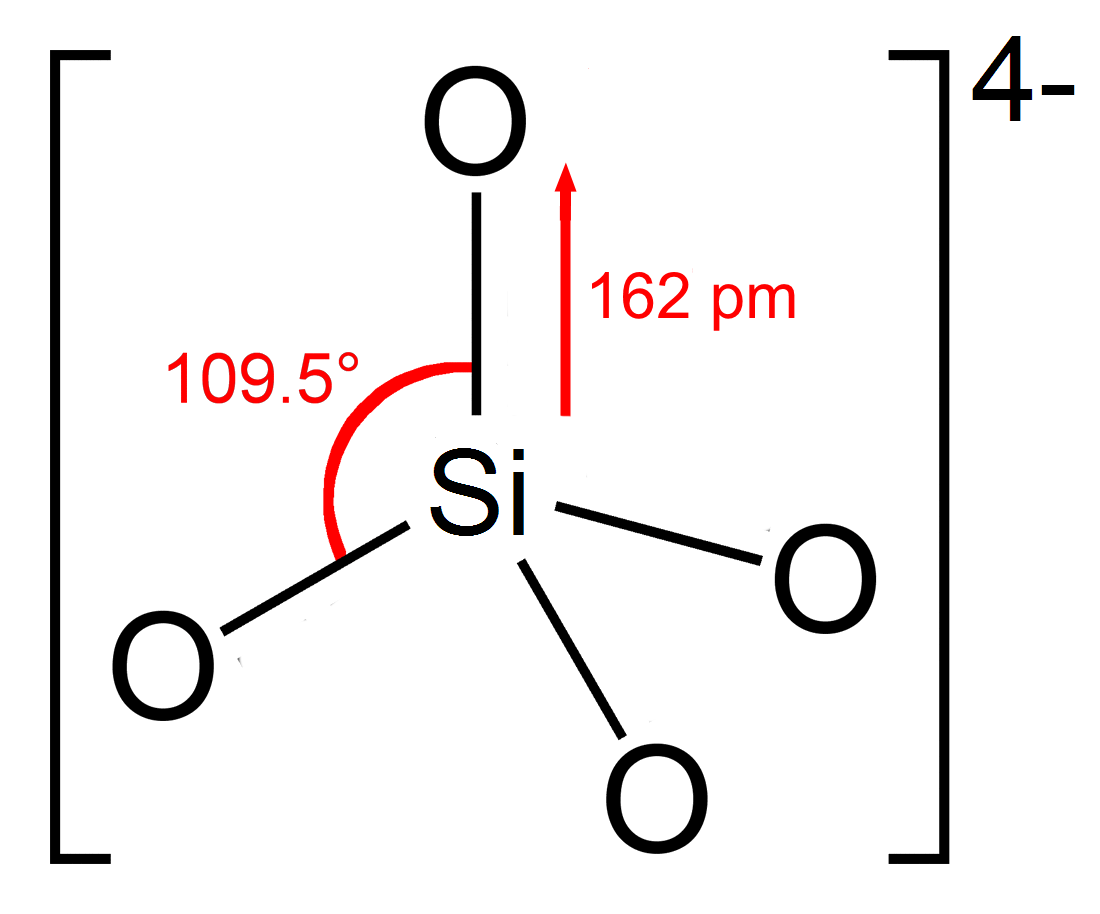
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |