|
Ethienocarb
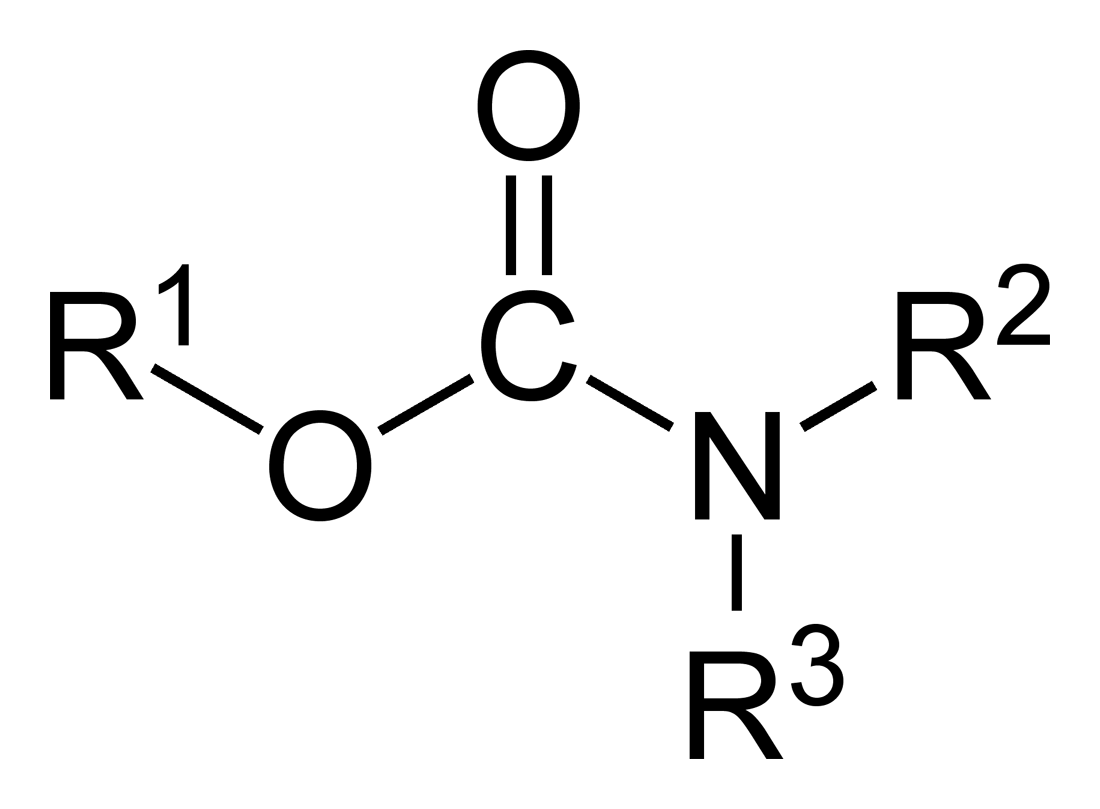
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). History and etymology Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant. Nomenclature According to IUPAC, carbon atoms forming valine are numbered sequentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C-N=C angle is 134.9° and the N=C=O angle is 173.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curtius Rearrangement
The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published. Preparation of acyl azide The acyl azide is usually made from the reaction of acid chlorides or anydrides with sodium azide or trimethylsilyl azide. Acyl azides are also obtained from treating acylhydrazines with nitrous acid. Alternatively, the acyl azide can be formed by the direct reaction of a carboxylic acid with diphenylphosphoryl azide (DPPA). Reaction mechanism It was believed that the Curtius rearrangement was a two-step processes, with the loss of nitrogen gas forming an acyl nitrene, followed by migration of the R-group to give the isocyanate. However, recent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ... atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic compound, Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroformate
Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available. Chloroformates are used as reagents in organic chemistry. For example, benzyl chloroformate is used to introduce the Cbz (carboxybenzyl) protecting group and fluorenylmethyloxycarbonyl chloride is used to introduce the FMOC protecting group. Chloroformates are popular in the field of chromatography as derivatization agents. They convert polar compounds into less polar more volatile derivatives. In this way, chloroformates enable relatively simple transformation of large array of metabolites (aminoacids, amines, carboxylic acids, phenols) for analysis by gas chromatography / mass spectrometry. Reactions The reactivity of chloroformates and acyl chlorides are similar. Representative reactions are: * Reaction with ami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
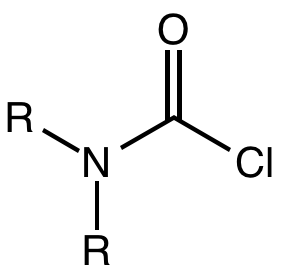
Carbamoyl Chloride
A carbamoyl chloride is the functional group with the formula R2NC(O)Cl. The parent carbamoyl chloride, H2NCOCl is unstable, but many N-substituted analogues are known. Most examples are moisture sensitive, colourless, and soluble in nonpolar organic solvents. An example is dimethylcarbamoyl chloride (m.p. −90 °C and b.p. 93 °C). Carbamoyl chlorides are used to prepare a number of pesticides, e.g. carbofuran and aldicarb. Production and examples Carbamoyl chlorides are prepared by the reaction of an amine with phosgene: :2 R2NH + COCl2 → R2NCOCl + 2NH2l They also arise by the addition of hydrogen chloride to isocyanates: :RNCO + HCl → RNHCOCl In this way, carbamonyl chlorides can be prepared with N-H functionality. Reactions In a reaction that is typically avoided, hydrolysis of carbamoyl chlorides gives carbamic acids: :R2NCOCl + H2O → R2NC(O)OH + HCl Owing to the influence of the amino group, these compounds are less hydrolytically sensitive than the usual acid ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





-3D-balls.png)