quantum physics on:
[Wikipedia]
[Google]
[Amazon]
 Quantum mechanics is the fundamental physical
Quantum mechanics is the fundamental physical
 Another consequence of the mathematical rules of quantum mechanics is the phenomenon of quantum interference, which is often illustrated with the
Another consequence of the mathematical rules of quantum mechanics is the phenomenon of quantum interference, which is often illustrated with the  Another non-classical phenomenon predicted by quantum mechanics is
Another non-classical phenomenon predicted by quantum mechanics is
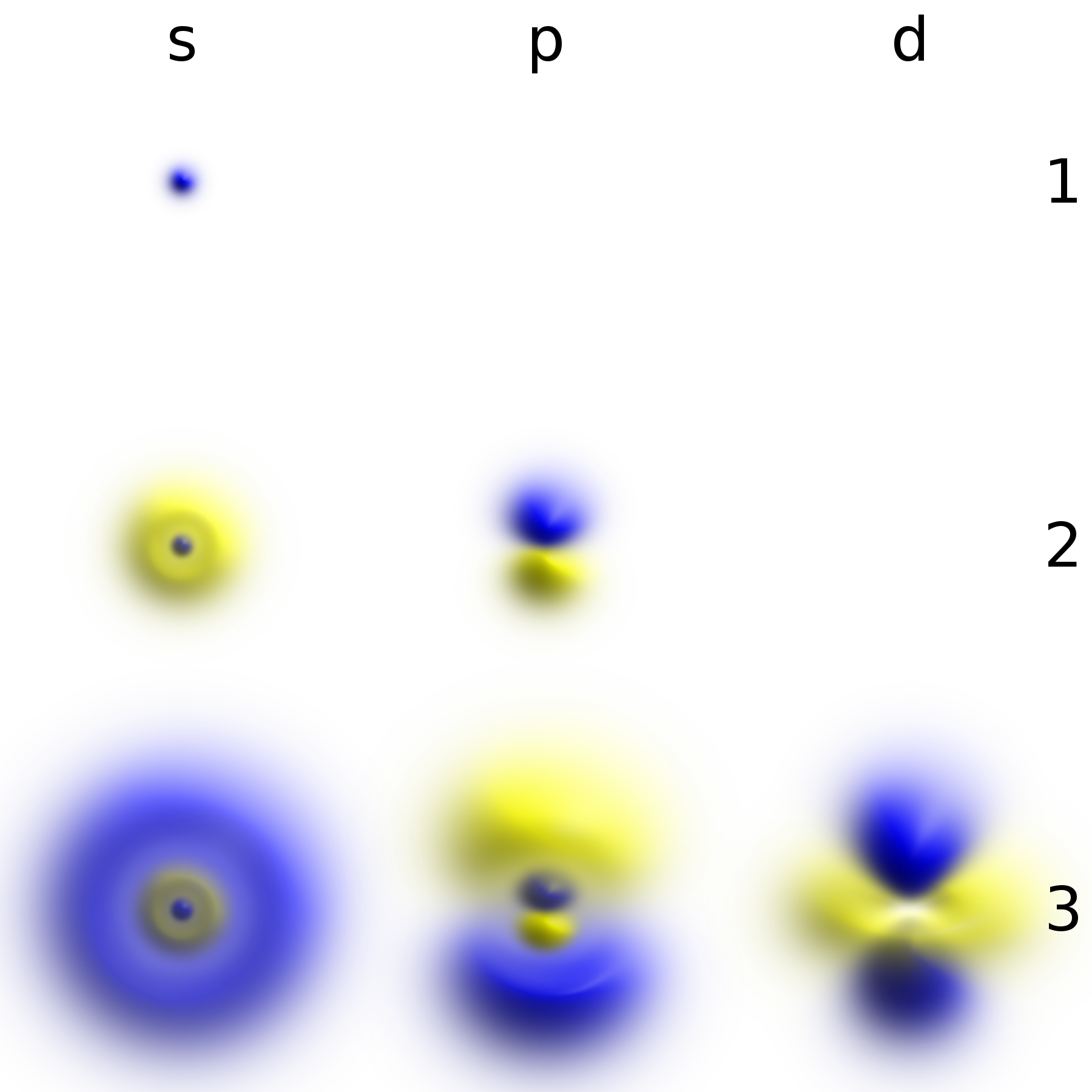 Some wave functions produce probability distributions that are independent of time, such as
Some wave functions produce probability distributions that are independent of time, such as
 The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible
The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible
 The particle in a one-dimensional potential energy box is the most mathematically simple example where restraints lead to the quantization of energy levels. The box is defined as having zero potential energy everywhere inside a certain region, and therefore infinite potential energy everywhere outside that region. For the one-dimensional case in the direction, the time-independent Schrödinger equation may be written
With the differential operator defined by
the previous equation is evocative of the classic kinetic energy analogue,
with state in this case having energy coincident with the kinetic energy of the particle.
The general solutions of the Schrödinger equation for the particle in a box are
or, from
The particle in a one-dimensional potential energy box is the most mathematically simple example where restraints lead to the quantization of energy levels. The box is defined as having zero potential energy everywhere inside a certain region, and therefore infinite potential energy everywhere outside that region. For the one-dimensional case in the direction, the time-independent Schrödinger equation may be written
With the differential operator defined by
the previous equation is evocative of the classic kinetic energy analogue,
with state in this case having energy coincident with the kinetic energy of the particle.
The general solutions of the Schrödinger equation for the particle in a box are
or, from
 As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
 The Mach–Zehnder interferometer (MZI) illustrates the concepts of superposition and interference with linear algebra in dimension 2, rather than differential equations. It can be seen as a simplified version of the double-slit experiment, but it is of interest in its own right, for example in the delayed choice quantum eraser, the Elitzur–Vaidman bomb tester, and in studies of quantum entanglement.
We can model a photon going through the interferometer by considering that at each point it can be in a superposition of only two paths: the "lower" path which starts from the left, goes straight through both beam splitters, and ends at the top, and the "upper" path which starts from the bottom, goes straight through both beam splitters, and ends at the right. The quantum state of the photon is therefore a vector that is a superposition of the "lower" path and the "upper" path , that is, for complex . In order to respect the postulate that we require that .
Both
The Mach–Zehnder interferometer (MZI) illustrates the concepts of superposition and interference with linear algebra in dimension 2, rather than differential equations. It can be seen as a simplified version of the double-slit experiment, but it is of interest in its own right, for example in the delayed choice quantum eraser, the Elitzur–Vaidman bomb tester, and in studies of quantum entanglement.
We can model a photon going through the interferometer by considering that at each point it can be in a superposition of only two paths: the "lower" path which starts from the left, goes straight through both beam splitters, and ends at the top, and the "upper" path which starts from the bottom, goes straight through both beam splitters, and ends at the right. The quantum state of the photon is therefore a vector that is a superposition of the "lower" path and the "upper" path , that is, for complex . In order to respect the postulate that we require that .
Both
 Everett's
Everett's
 This phase is known as the
This phase is known as the
Draft of 4th edition.
*
Online copy
* * Gunther Ludwig, 1968. ''Wave Mechanics''. London: Pergamon Press. * George Mackey (2004). ''The mathematical foundations of quantum mechanics''. Dover Publications. . * * Albert Messiah, 1966. ''Quantum Mechanics'' (Vol. I), English translation from French by G. M. Temmer. North Holland, John Wiley & Sons. Cf. chpt. IV, section III
online
* * Considers the extent to which chemistry and the periodic system have been reduced to quantum mechanics. * * * * * Veltman, Martinus J. G. (2003), ''Facts and Mysteries in Elementary Particle Physics''.
Introduction to Quantum Theory at Quantiki.
Quantum Physics Made Relatively Simple
three video lectures by Hans Bethe. Course material
Quantum Cook Book
an
PHYS 201: Fundamentals of Physics II
by Ramamurti Shankar, Yale OpenCourseware. *
Modern Physics: With waves, thermodynamics, and optics
' – an online textbook. *
Chemistry
an
Physics
Se
8.04
an
Examples in Quantum Mechanics
Philosophy * * {{Authority control
 Quantum mechanics is the fundamental physical
Quantum mechanics is the fundamental physical theory
A theory is a systematic and rational form of abstract thinking about a phenomenon, or the conclusions derived from such thinking. It involves contemplative and logical reasoning, often supported by processes such as observation, experimentation, ...
that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
, quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
, quantum technology, and quantum information science
Quantum information science is a field that combines the principles of quantum mechanics with information theory to study the processing, analysis, and transmission of information. It covers both theoretical and experimental aspects of quantum phys ...
.
Quantum mechanics can describe many systems that classical physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to ...
cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenome ...
and (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales.
Quantum systems have bound states that are quantized to discrete values of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
, momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
, angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
, and other quantities, in contrast to classical systems where these quantities can be measured continuously. Measurements of quantum systems show characteristics of both particle
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
s and wave
In physics, mathematics, engineering, and related fields, a wave is a propagating dynamic disturbance (change from List of types of equilibrium, equilibrium) of one or more quantities. ''Periodic waves'' oscillate repeatedly about an equilibrium ...
s (wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
), and there are limits to how accurately the value of a physical quantity can be predicted prior to its measurement, given a complete set of initial conditions (the uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
).
Quantum mechanics arose gradually from theories to explain observations that could not be reconciled with classical physics
Classical physics refers to physics theories that are non-quantum or both non-quantum and non-relativistic, depending on the context. In historical discussions, ''classical physics'' refers to pre-1900 physics, while '' modern physics'' refers to ...
, such as Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918.
Planck made many substantial con ...
's solution in 1900 to the black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
problem, and the correspondence between energy and frequency in Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
's 1905 paper, which explained the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
. These early attempts to understand microscopic phenomena, now known as the "old quantum theory
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The th ...
", led to the full development of quantum mechanics in the mid-1920s by Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
, Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
, Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
, Paul Dirac
Paul Adrien Maurice Dirac ( ; 8 August 1902 – 20 October 1984) was an English mathematician and Theoretical physics, theoretical physicist who is considered to be one of the founders of quantum mechanics. Dirac laid the foundations for bot ...
and others. The modern theory is formulated in various specially developed mathematical formalisms. In one of them, a mathematical entity called the wave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
provides information, in the form of probability amplitude
In quantum mechanics, a probability amplitude is a complex number used for describing the behaviour of systems. The square of the modulus of this quantity at a point in space represents a probability density at that point.
Probability amplitu ...
s, about what measurements of a particle's energy, momentum, and other physical properties may yield.
Overview and fundamental concepts
Quantum mechanics allows the calculation of properties and behaviour of physical systems. It is typically applied to microscopic systems:molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
, atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
and subatomic particle
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a baryon, lik ...
s. It has been demonstrated to hold for complex molecules with thousands of atoms, but its application to human beings raises philosophical problems, such as Wigner's friend
Wigner's friend is a thought experiment in theoretical quantum physics, first published by the Hungarian-American physicist Eugene Wigner in 1961, Reprinted in and further developed by David Deutsch in 1985. The scenario involves an indirect obse ...
, and its application to the universe as a whole remains speculative. Predictions of quantum mechanics have been verified experimentally to an extremely high degree of accuracy
Accuracy and precision are two measures of ''observational error''.
''Accuracy'' is how close a given set of measurements (observations or readings) are to their ''true value''.
''Precision'' is how close the measurements are to each other.
The ...
. For example, the refinement of quantum mechanics for the interaction of light and matter, known as quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
(QED), has been shown to agree with experiment to within 1 part in 1012 when predicting the magnetic properties of an electron.
A fundamental feature of the theory is that it usually cannot predict with certainty what will happen, but only give probabilities. Mathematically, a probability is found by taking the square of the absolute value of a complex number
In mathematics, a complex number is an element of a number system that extends the real numbers with a specific element denoted , called the imaginary unit and satisfying the equation i^= -1; every complex number can be expressed in the for ...
, known as a probability amplitude. This is known as the Born rule
The Born rule is a postulate of quantum mechanics that gives the probability that a measurement of a quantum system will yield a given result. In one commonly used application, it states that the probability density for finding a particle at a ...
, named after physicist Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German-British theoretical physicist who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics, and supervised the work of a ...
. For example, a quantum particle like an electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
can be described by a wave function, which associates to each point in space a probability amplitude. Applying the Born rule to these amplitudes gives a probability density function
In probability theory, a probability density function (PDF), density function, or density of an absolutely continuous random variable, is a Function (mathematics), function whose value at any given sample (or point) in the sample space (the s ...
for the position that the electron will be found to have when an experiment is performed to measure it. This is the best the theory can do; it cannot say for certain where the electron will be found. The Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
relates the collection of probability amplitudes that pertain to one moment of time to the collection of probability amplitudes that pertain to another.
One consequence of the mathematical rules of quantum mechanics is a tradeoff in predictability between measurable quantities. The most famous form of this uncertainty principle
The uncertainty principle, also known as Heisenberg's indeterminacy principle, is a fundamental concept in quantum mechanics. It states that there is a limit to the precision with which certain pairs of physical properties, such as position a ...
says that no matter how a quantum particle is prepared or how carefully experiments upon it are arranged, it is impossible to have a precise prediction for a measurement of its position and also at the same time for a measurement of its momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
.
double-slit experiment
In modern physics, the double-slit experiment demonstrates that light and matter can exhibit behavior of both classical particles and classical waves. This type of experiment was first performed by Thomas Young in 1801, as a demonstration of ...
. In the basic version of this experiment, a coherent light source, such as a laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
beam, illuminates a plate pierced by two parallel slits, and the light passing through the slits is observed on a screen behind the plate. The wave nature of light causes the light waves passing through the two slits to interfere, producing bright and dark bands on the screen – a result that would not be expected if light consisted of classical particles. However, the light is always found to be absorbed at the screen at discrete points, as individual particles rather than waves; the interference pattern appears via the varying density of these particle hits on the screen. Furthermore, versions of the experiment that include detectors at the slits find that each detected photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
passes through one slit (as would a classical particle), and not through both slits (as would a wave). However, such experiments demonstrate that particles do not form the interference pattern if one detects which slit they pass through. This behavior is known as wave–particle duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the in ...
. In addition to light, electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
, atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
, and molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
are all found to exhibit the same dual behavior when fired towards a double slit.
 Another non-classical phenomenon predicted by quantum mechanics is
Another non-classical phenomenon predicted by quantum mechanics is quantum tunnelling
In physics, quantum tunnelling, barrier penetration, or simply tunnelling is a quantum mechanical phenomenon in which an object such as an electron or atom passes through a potential energy barrier that, according to classical mechanics, shoul ...
: a particle that goes up against a potential barrier can cross it, even if its kinetic energy is smaller than the maximum of the potential. In classical mechanics this particle would be trapped. Quantum tunnelling has several important consequences, enabling radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
in stars, and applications such as scanning tunnelling microscopy, tunnel diode
A tunnel diode or Esaki diode is a type of semiconductor diode that has effectively " negative resistance" due to the quantum mechanical effect called tunneling. It was invented in August 1957 by Leo Esaki and Yuriko Kurose when working ...
and tunnel field-effect transistor
The tunnel field-effect transistor (TFET) is an experimental type of transistor. Even though its structure is very similar to a metal–oxide–semiconductor field-effect transistor (MOSFET), the fundamental switching mechanism differs, making this ...
.
When quantum systems interact, the result can be the creation of quantum entanglement
Quantum entanglement is the phenomenon where the quantum state of each Subatomic particle, particle in a group cannot be described independently of the state of the others, even when the particles are separated by a large distance. The topic o ...
: their properties become so intertwined that a description of the whole solely in terms of the individual parts is no longer possible. Erwin Schrödinger called entanglement "...the characteristic trait of quantum mechanics, the one that enforces its entire departure from classical lines of thought". Quantum entanglement enables quantum computing
A quantum computer is a computer that exploits quantum mechanical phenomena. On small scales, physical matter exhibits properties of wave-particle duality, both particles and waves, and quantum computing takes advantage of this behavior using s ...
and is part of quantum communication protocols, such as quantum key distribution
Quantum key distribution (QKD) is a secure communication method that implements a cryptographic protocol involving components of quantum mechanics. It enables two parties to produce a shared random secret key known only to them, which then can b ...
and superdense coding
In quantum information theory, superdense coding (also referred to as ''dense coding'') is a quantum communication protocol to communicate a number of classical bits of information by only transmitting a smaller number of qubits, under the ass ...
. Contrary to popular misconception, entanglement does not allow sending signals faster than light
Faster-than-light (superluminal or supercausal) travel and communication are the conjectural propagation of matter or information faster than the speed of light in vacuum (). The special theory of relativity implies that only particles with zero ...
, as demonstrated by the no-communication theorem.
Another possibility opened by entanglement is testing for " hidden variables", hypothetical properties more fundamental than the quantities addressed in quantum theory itself, knowledge of which would allow more exact predictions than quantum theory provides. A collection of results, most significantly Bell's theorem
Bell's theorem is a term encompassing a number of closely related results in physics, all of which determine that quantum mechanics is incompatible with local hidden-variable theories, given some basic assumptions about the nature of measuremen ...
, have demonstrated that broad classes of such hidden-variable theories are in fact incompatible with quantum physics. According to Bell's theorem, if nature actually operates in accord with any theory of local hidden variables, then the results of a Bell test will be constrained in a particular, quantifiable way. Many Bell tests have been performed and they have shown results incompatible with the constraints imposed by local hidden variables.
It is not possible to present these concepts in more than a superficial way without introducing the mathematics involved; understanding quantum mechanics requires not only manipulating complex numbers, but also linear algebra
Linear algebra is the branch of mathematics concerning linear equations such as
:a_1x_1+\cdots +a_nx_n=b,
linear maps such as
:(x_1, \ldots, x_n) \mapsto a_1x_1+\cdots +a_nx_n,
and their representations in vector spaces and through matrix (mathemat ...
, differential equations, group theory
In abstract algebra, group theory studies the algebraic structures known as group (mathematics), groups.
The concept of a group is central to abstract algebra: other well-known algebraic structures, such as ring (mathematics), rings, field ( ...
, and other more advanced subjects. Accordingly, this article will present a mathematical formulation of quantum mechanics and survey its application to some useful and oft-studied examples.
Mathematical formulation
In the mathematically rigorous formulation of quantum mechanics, the state of a quantum mechanical system is a vector belonging to a ( separable) complexHilbert space
In mathematics, a Hilbert space is a real number, real or complex number, complex inner product space that is also a complete metric space with respect to the metric induced by the inner product. It generalizes the notion of Euclidean space. The ...
. This vector is postulated to be normalized under the Hilbert space inner product, that is, it obeys , and it is well-defined up to a complex number of modulus 1 (the global phase), that is, and represent the same physical system. In other words, the possible states are points in the projective space
In mathematics, the concept of a projective space originated from the visual effect of perspective, where parallel lines seem to meet ''at infinity''. A projective space may thus be viewed as the extension of a Euclidean space, or, more generally ...
of a Hilbert space, usually called the complex projective space
In mathematics, complex projective space is the projective space with respect to the field of complex numbers. By analogy, whereas the points of a real projective space label the lines through the origin of a real Euclidean space, the points of a ...
. The exact nature of this Hilbert space is dependent on the system – for example, for describing position and momentum the Hilbert space is the space of complex square-integrable functions , while the Hilbert space for the spin of a single proton is simply the space of two-dimensional complex vectors with the usual inner product.
Physical quantities of interestposition, momentum, energy, spinare represented by observables, which are Hermitian (more precisely, self-adjoint
In mathematics, an element of a *-algebra is called self-adjoint if it is the same as its adjoint (i.e. a = a^*).
Definition
Let \mathcal be a *-algebra. An element a \in \mathcal is called self-adjoint if
The set of self-adjoint elements ...
) linear operators acting on the Hilbert space. A quantum state can be an eigenvector
In linear algebra, an eigenvector ( ) or characteristic vector is a vector that has its direction unchanged (or reversed) by a given linear transformation. More precisely, an eigenvector \mathbf v of a linear transformation T is scaled by ...
of an observable, in which case it is called an eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
, and the associated eigenvalue
In linear algebra, an eigenvector ( ) or characteristic vector is a vector that has its direction unchanged (or reversed) by a given linear transformation. More precisely, an eigenvector \mathbf v of a linear transformation T is scaled by a ...
corresponds to the value of the observable in that eigenstate. More generally, a quantum state will be a linear combination of the eigenstates, known as a quantum superposition
Quantum superposition is a fundamental principle of quantum mechanics that states that linear combinations of solutions to the Schrödinger equation are also solutions of the Schrödinger equation. This follows from the fact that the Schrödi ...
. When an observable is measured, the result will be one of its eigenvalues with probability given by the Born rule
The Born rule is a postulate of quantum mechanics that gives the probability that a measurement of a quantum system will yield a given result. In one commonly used application, it states that the probability density for finding a particle at a ...
: in the simplest case the eigenvalue is non-degenerate and the probability is given by , where is its associated unit-length eigenvector. More generally, the eigenvalue is degenerate and the probability is given by , where is the projector onto its associated eigenspace. In the continuous case, these formulas give instead the probability density.
After the measurement, if result was obtained, the quantum state is postulated to collapse to , in the non-degenerate case, or to , in the general case. The probabilistic
Probability is a branch of mathematics and statistics concerning events and numerical descriptions of how likely they are to occur. The probability of an event is a number between 0 and 1; the larger the probability, the more likely an e ...
nature of quantum mechanics thus stems from the act of measurement. This is one of the most difficult aspects of quantum systems to understand. It was the central topic in the famous Bohr–Einstein debates, in which the two scientists attempted to clarify these fundamental principles by way of thought experiment
A thought experiment is an imaginary scenario that is meant to elucidate or test an argument or theory. It is often an experiment that would be hard, impossible, or unethical to actually perform. It can also be an abstract hypothetical that is ...
s. In the decades after the formulation of quantum mechanics, the question of what constitutes a "measurement" has been extensively studied. Newer interpretations of quantum mechanics
An interpretation of quantum mechanics is an attempt to explain how the mathematical theory of quantum mechanics might correspond to experienced reality. Quantum mechanics has held up to rigorous and extremely precise tests in an extraordinarily b ...
have been formulated that do away with the concept of " wave function collapse" (see, for example, the many-worlds interpretation
The many-worlds interpretation (MWI) is an interpretation of quantum mechanics that asserts that the universal wavefunction is Philosophical realism, objectively real, and that there is no wave function collapse. This implies that all Possible ...
). The basic idea is that when a quantum system interacts with a measuring apparatus, their respective wave functions become entangled so that the original quantum system ceases to exist as an independent entity (see '' Measurement in quantum mechanics'').
Time evolution of a quantum state
The time evolution of a quantum state is described by the Schrödinger equation: Here denotes theHamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
, the observable corresponding to the total energy of the system, and is the reduced Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
. The constant is introduced so that the Hamiltonian is reduced to the classical Hamiltonian in cases where the quantum system can be approximated by a classical system; the ability to make such an approximation in certain limits is called the correspondence principle
In physics, a correspondence principle is any one of several premises or assertions about the relationship between classical and quantum mechanics.
The physicist Niels Bohr coined the term in 1920 during the early development of quantum theory; ...
.
The solution of this differential equation is given by
The operator is known as the time-evolution operator, and has the crucial property that it is unitary. This time evolution is deterministic
Determinism is the metaphysical view that all events within the universe (or multiverse) can occur only in one possible way. Deterministic theories throughout the history of philosophy have developed from diverse and sometimes overlapping mo ...
in the sense that – given an initial quantum state – it makes a definite prediction of what the quantum state will be at any later time.
 Some wave functions produce probability distributions that are independent of time, such as
Some wave functions produce probability distributions that are independent of time, such as eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
s of the Hamiltonian. Many systems that are treated dynamically in classical mechanics are described by such "static" wave functions. For example, a single electron in an unexcited atom is pictured classically as a particle moving in a circular trajectory around the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
, whereas in quantum mechanics, it is described by a static wave function surrounding the nucleus. For example, the electron wave function for an unexcited hydrogen atom is a spherically symmetric function known as an ''s'' orbital ( Fig. 1).
Analytic solutions of the Schrödinger equation are known for very few relatively simple model Hamiltonians including the quantum harmonic oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, ...
, the particle in a box, the dihydrogen cation, and the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb for ...
. Even the helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
atom – which contains just two electrons – has defied all attempts at a fully analytic treatment, admitting no solution in closed form.
However, there are techniques for finding approximate solutions. One method, called perturbation theory
In mathematics and applied mathematics, perturbation theory comprises methods for finding an approximate solution to a problem, by starting from the exact solution of a related, simpler problem. A critical feature of the technique is a middle ...
, uses the analytic result for a simple quantum mechanical model to create a result for a related but more complicated model by (for example) the addition of a weak potential energy
In physics, potential energy is the energy of an object or system due to the body's position relative to other objects, or the configuration of its particles. The energy is equal to the work done against any restoring forces, such as gravity ...
. Another approximation method applies to systems for which quantum mechanics produces only small deviations from classical behavior. These deviations can then be computed based on the classical motion.
Uncertainty principle
One consequence of the basic quantum formalism is the uncertainty principle. In its most familiar form, this states that no preparation of a quantum particle can imply simultaneously precise predictions both for a measurement of its position and for a measurement of its momentum. Both position and momentum are observables, meaning that they are represented by Hermitian operators. The position operator and momentum operator do not commute, but rather satisfy thecanonical commutation relation
In quantum mechanics, the canonical commutation relation is the fundamental relation between canonical conjugate quantities (quantities which are related by definition such that one is the Fourier transform of another). For example,
hat x,\hat p ...
:
Given a quantum state, the Born rule lets us compute expectation values for both and , and moreover for powers of them. Defining the uncertainty for an observable by a standard deviation
In statistics, the standard deviation is a measure of the amount of variation of the values of a variable about its Expected value, mean. A low standard Deviation (statistics), deviation indicates that the values tend to be close to the mean ( ...
, we have
and likewise for the momentum:
The uncertainty principle states that
Either standard deviation can in principle be made arbitrarily small, but not both simultaneously.Section 3.2 of . This fact is experimentally well-known for example in quantum optics; see e.g. chap. 2 and Fig. 2.1 . This inequality generalizes to arbitrary pairs of self-adjoint operators and . The commutator
In mathematics, the commutator gives an indication of the extent to which a certain binary operation fails to be commutative. There are different definitions used in group theory and ring theory.
Group theory
The commutator of two elements, ...
of these two operators is
and this provides the lower bound on the product of standard deviations:
Another consequence of the canonical commutation relation is that the position and momentum operators are Fourier transforms of each other, so that a description of an object according to its momentum is the Fourier transform of its description according to its position. The fact that dependence in momentum is the Fourier transform of the dependence in position means that the momentum operator is equivalent (up to an factor) to taking the derivative according to the position, since in Fourier analysis differentiation corresponds to multiplication in the dual space. This is why in quantum equations in position space, the momentum is replaced by , and in particular in the non-relativistic Schrödinger equation in position space the momentum-squared term is replaced with a Laplacian times .
Composite systems and entanglement
When two different quantum systems are considered together, the Hilbert space of the combined system is thetensor product
In mathematics, the tensor product V \otimes W of two vector spaces V and W (over the same field) is a vector space to which is associated a bilinear map V\times W \rightarrow V\otimes W that maps a pair (v,w),\ v\in V, w\in W to an element of ...
of the Hilbert spaces of the two components. For example, let and be two quantum systems, with Hilbert spaces and , respectively. The Hilbert space of the composite system is then
If the state for the first system is the vector and the state for the second system is , then the state of the composite system is
Not all states in the joint Hilbert space can be written in this form, however, because the superposition principle implies that linear combinations of these "separable" or "product states" are also valid. For example, if and are both possible states for system , and likewise and are both possible states for system , then
is a valid joint state that is not separable. States that are not separable are called entangled.
If the state for a composite system is entangled, it is impossible to describe either component system or system by a state vector. One can instead define reduced density matrices that describe the statistics that can be obtained by making measurements on either component system alone. This necessarily causes a loss of information, though: knowing the reduced density matrices of the individual systems is not enough to reconstruct the state of the composite system. Just as density matrices specify the state of a subsystem of a larger system, analogously, positive operator-valued measures (POVMs) describe the effect on a subsystem of a measurement performed on a larger system. POVMs are extensively used in quantum information theory.
As described above, entanglement is a key feature of models of measurement processes in which an apparatus becomes entangled with the system being measured. Systems interacting with the environment in which they reside generally become entangled with that environment, a phenomenon known as quantum decoherence
Quantum decoherence is the loss of quantum coherence. It involves generally a loss of information of a system to its environment. Quantum decoherence has been studied to understand how quantum systems convert to systems that can be expla ...
. This can explain why, in practice, quantum effects are difficult to observe in systems larger than microscopic.
Equivalence between formulations
There are many mathematically equivalent formulations of quantum mechanics. One of the oldest and most common is the " transformation theory" proposed byPaul Dirac
Paul Adrien Maurice Dirac ( ; 8 August 1902 – 20 October 1984) was an English mathematician and Theoretical physics, theoretical physicist who is considered to be one of the founders of quantum mechanics. Dirac laid the foundations for bot ...
, which unifies and generalizes the two earliest formulations of quantum mechanics – matrix mechanics (invented by Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
) and wave mechanics (invented by Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
). An alternative formulation of quantum mechanics is Feynman's path integral formulation
The path integral formulation is a description in quantum mechanics that generalizes the stationary action principle of classical mechanics. It replaces the classical notion of a single, unique classical trajectory for a system with a sum, or ...
, in which a quantum-mechanical amplitude is considered as a sum over all possible classical and non-classical paths between the initial and final states. This is the quantum-mechanical counterpart of the action principle in classical mechanics.
Symmetries and conservation laws
The Hamiltonian is known as the ''generator'' of time evolution, since it defines a unitary time-evolution operator for each value of . From this relation between and , it follows that any observable that commutes with will be conserved: its expectation value will not change over time. This statement generalizes, as mathematically, any Hermitian operator can generate a family of unitary operators parameterized by a variable . Under the evolution generated by , any observable that commutes with will be conserved. Moreover, if is conserved by evolution under , then is conserved under the evolution generated by . This implies a quantum version of the result proven byEmmy Noether
Amalie Emmy Noether (23 March 1882 – 14 April 1935) was a German mathematician who made many important contributions to abstract algebra. She also proved Noether's theorem, Noether's first and Noether's second theorem, second theorems, which ...
in classical ( Lagrangian) mechanics: for every differentiable
In mathematics, a differentiable function of one real variable is a function whose derivative exists at each point in its domain. In other words, the graph of a differentiable function has a non- vertical tangent line at each interior point in ...
symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
of a Hamiltonian, there exists a corresponding conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves over time. Exact conservation laws include conservation of mass-energy, conservation of linear momen ...
.
Examples
Free particle
 The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible
The simplest example of a quantum system with a position degree of freedom is a free particle in a single spatial dimension. A free particle is one which is not subject to external influences, so that its Hamiltonian consists only of its kinetic energy:
The general solution of the Schrödinger equation is given by
which is a superposition of all possible plane wave
In physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of ...
s , which are eigenstates of the momentum operator with momentum . The coefficients of the superposition are , which is the Fourier transform of the initial quantum state .
It is not possible for the solution to be a single momentum eigenstate, or a single position eigenstate, as these are not normalizable quantum states. Instead, we can consider a Gaussian wave packet
In physics, a wave packet (also known as a wave train or wave group) is a short burst of localized wave action that travels as a unit, outlined by an Envelope (waves), envelope. A wave packet can be analyzed into, or can be synthesized from, a ...
:
which has Fourier transform, and therefore momentum distribution
We see that as we make smaller the spread in position gets smaller, but the spread in momentum gets larger. Conversely, by making larger we make the spread in momentum smaller, but the spread in position gets larger. This illustrates the uncertainty principle.
As we let the Gaussian wave packet evolve in time, we see that its center moves through space at a constant velocity (like a classical particle with no forces acting on it). However, the wave packet will also spread out as time progresses, which means that the position becomes more and more uncertain. The uncertainty in momentum, however, stays constant.
Particle in a box
Euler's formula
Euler's formula, named after Leonhard Euler, is a mathematical formula in complex analysis that establishes the fundamental relationship between the trigonometric functions and the complex exponential function. Euler's formula states that, for ...
,
The infinite potential walls of the box determine the values of and at and where must be zero. Thus, at ,
and . At ,
in which cannot be zero as this would conflict with the postulate that has norm 1. Therefore, since , must be an integer multiple of ,
This constraint on implies a constraint on the energy levels, yielding
A finite potential well is the generalization of the infinite potential well problem to potential wells having finite depth. The finite potential well problem is mathematically more complicated than the infinite particle-in-a-box problem as the wave function is not pinned to zero at the walls of the well. Instead, the wave function must satisfy more complicated mathematical boundary conditions as it is nonzero in regions outside the well. Another related problem is that of the rectangular potential barrier, which furnishes a model for the quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
effect that plays an important role in the performance of modern technologies such as flash memory
Flash memory is an Integrated circuit, electronic Non-volatile memory, non-volatile computer memory storage medium that can be electrically erased and reprogrammed. The two main types of flash memory, NOR flash and NAND flash, are named for t ...
and scanning tunneling microscopy
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
.
Harmonic oscillator
 As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The
As in the classical case, the potential for the quantum harmonic oscillator is given by
This problem can either be treated by directly solving the Schrödinger equation, which is not trivial, or by using the more elegant "ladder method" first proposed by Paul Dirac. The eigenstate
In quantum physics, a quantum state is a mathematical entity that embodies the knowledge of a quantum system. Quantum mechanics specifies the construction, evolution, and measurement of a quantum state. The result is a prediction for the system re ...
s are given by
where ''Hn'' are the Hermite polynomials
In mathematics, the Hermite polynomials are a classical orthogonal polynomial sequence.
The polynomials arise in:
* signal processing as Hermitian wavelets for wavelet transform analysis
* probability, such as the Edgeworth series, as well a ...
and the corresponding energy levels are
This is another example illustrating the discretization of energy for bound state
A bound state is a composite of two or more fundamental building blocks, such as particles, atoms, or bodies, that behaves as a single object and in which energy is required to split them.
In quantum physics, a bound state is a quantum state of a ...
s.
Mach–Zehnder interferometer
beam splitter
A beam splitter or beamsplitter is an optical instrument, optical device that splits a beam of light into a transmitted and a reflected beam. It is a crucial part of many optical experimental and measurement systems, such as Interferometry, int ...
s are modelled as the unitary matrix , which means that when a photon meets the beam splitter it will either stay on the same path with a probability amplitude of , or be reflected to the other path with a probability amplitude of . The phase shifter on the upper arm is modelled as the unitary matrix , which means that if the photon is on the "upper" path it will gain a relative phase of , and it will stay unchanged if it is in the lower path.
A photon that enters the interferometer from the left will then be acted upon with a beam splitter , a phase shifter , and another beam splitter , and so end up in the state
and the probabilities that it will be detected at the right or at the top are given respectively by
One can therefore use the Mach–Zehnder interferometer to estimate the phase shift
In physics and mathematics, the phase (symbol φ or ϕ) of a wave or other periodic function F of some real variable t (such as time) is an angle-like quantity representing the fraction of the cycle covered up to t. It is expressed in such a s ...
by estimating these probabilities.
It is interesting to consider what would happen if the photon were definitely in either the "lower" or "upper" paths between the beam splitters. This can be accomplished by blocking one of the paths, or equivalently by removing the first beam splitter (and feeding the photon from the left or the bottom, as desired). In both cases, there will be no interference between the paths anymore, and the probabilities are given by , independently of the phase . From this we can conclude that the photon does not take one path or another after the first beam splitter, but rather that it is in a genuine quantum superposition of the two paths.
Applications
Quantum mechanics has had enormous success in explaining many of the features of our universe, with regard to small-scale and discrete quantities and interactions which cannot be explained by classical methods. Quantum mechanics is often the only theory that can reveal the individual behaviors of the subatomic particles that make up all forms of matter (electrons,proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s, neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s, photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, and others). Solid-state physics
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state phy ...
and materials science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.
The intellectual origins of materials sci ...
are dependent upon quantum mechanics.
In many aspects, modern technology operates at a scale where quantum effects are significant. Important applications of quantum theory include quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
, quantum optics
Quantum optics is a branch of atomic, molecular, and optical physics and quantum chemistry that studies the behavior of photons (individual quanta of light). It includes the study of the particle-like properties of photons and their interaction ...
, quantum computing
A quantum computer is a computer that exploits quantum mechanical phenomena. On small scales, physical matter exhibits properties of wave-particle duality, both particles and waves, and quantum computing takes advantage of this behavior using s ...
, superconducting magnet
A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire has no electrical resistance and therefore can conduct much ...
s, light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corre ...
s, the optical amplifier
An optical amplifier is a device that amplifies an optical signal directly, without the need to first convert it to an electrical signal. An optical amplifier may be thought of as a laser without an optical cavity, or one in which feedback fro ...
and the laser, the transistor
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch electrical signals and electric power, power. It is one of the basic building blocks of modern electronics. It is composed of semicondu ...
and semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s such as the microprocessor
A microprocessor is a computer processor (computing), processor for which the data processing logic and control is included on a single integrated circuit (IC), or a small number of ICs. The microprocessor contains the arithmetic, logic, a ...
, medical and research imaging such as magnetic resonance imaging
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to generate pictures of the anatomy and the physiological processes inside the body. MRI scanners use strong magnetic fields, magnetic field gradients, and ...
and electron microscopy
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing i ...
. Explanations for many biological and physical phenomena are rooted in the nature of the chemical bond, most notably the macro-molecule DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
.
Relation to other scientific theories
Classical mechanics
The rules of quantum mechanics assert that the state space of a system is a Hilbert space and that observables of the system are Hermitian operators acting on vectors in that space – although they do not tell us which Hilbert space or which operators. These can be chosen appropriately in order to obtain a quantitative description of a quantum system, a necessary step in making physical predictions. An important guide for making these choices is thecorrespondence principle
In physics, a correspondence principle is any one of several premises or assertions about the relationship between classical and quantum mechanics.
The physicist Niels Bohr coined the term in 1920 during the early development of quantum theory; ...
, a heuristic which states that the predictions of quantum mechanics reduce to those of classical mechanics
Classical mechanics is a Theoretical physics, physical theory describing the motion of objects such as projectiles, parts of Machine (mechanical), machinery, spacecraft, planets, stars, and galaxies. The development of classical mechanics inv ...
in the regime of large quantum number
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.
To fully specify the state of the electron in a hydrogen atom, four quantum numbers are needed. The traditional set of quantu ...
s. One can also start from an established classical model of a particular system, and then try to guess the underlying quantum model that would give rise to the classical model in the correspondence limit. This approach is known as quantization.
When quantum mechanics was originally formulated, it was applied to models whose correspondence limit was non-relativistic classical mechanics. For instance, the well-known model of the quantum harmonic oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, ...
uses an explicitly non-relativistic expression for the kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
of the oscillator, and is thus a quantum version of the classical harmonic oscillator.
Complications arise with chaotic systems, which do not have good quantum numbers, and quantum chaos studies the relationship between classical and quantum descriptions in these systems.
Quantum decoherence
Quantum decoherence is the loss of quantum coherence. It involves generally a loss of information of a system to its environment. Quantum decoherence has been studied to understand how quantum systems convert to systems that can be expla ...
is a mechanism through which quantum systems lose coherence, and thus become incapable of displaying many typically quantum effects: quantum superposition
Quantum superposition is a fundamental principle of quantum mechanics that states that linear combinations of solutions to the Schrödinger equation are also solutions of the Schrödinger equation. This follows from the fact that the Schrödi ...
s become simply probabilistic mixtures, and quantum entanglement becomes simply classical correlations. Quantum coherence is not typically evident at macroscopic scales, though at temperatures approaching absolute zero
Absolute zero is the lowest possible temperature, a state at which a system's internal energy, and in ideal cases entropy, reach their minimum values. The absolute zero is defined as 0 K on the Kelvin scale, equivalent to −273.15 ° ...
quantum behavior may manifest macroscopically.
Many macroscopic properties of a classical system are a direct consequence of the quantum behavior of its parts. For example, the stability of bulk matter (consisting of atoms and molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s which would quickly collapse under electric forces alone), the rigidity of solids, and the mechanical, thermal, chemical, optical and magnetic properties of matter are all results of the interaction of electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
s under the rules of quantum mechanics.
Special relativity and electrodynamics
Early attempts to merge quantum mechanics withspecial relativity
In physics, the special theory of relativity, or special relativity for short, is a scientific theory of the relationship between Spacetime, space and time. In Albert Einstein's 1905 paper, Annus Mirabilis papers#Special relativity,
"On the Ele ...
involved the replacement of the Schrödinger equation with a covariant equation such as the Klein–Gordon equation or the Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-1/2 massive particles, called "Dirac ...
. While these theories were successful in explaining many experimental results, they had certain unsatisfactory qualities stemming from their neglect of the relativistic creation and annihilation of particles. A fully relativistic quantum theory required the development of quantum field theory, which applies quantization to a field (rather than a fixed set of particles). The first complete quantum field theory, quantum electrodynamics
In particle physics, quantum electrodynamics (QED) is the Theory of relativity, relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quant ...
, provides a fully quantum description of the electromagnetic interaction. Quantum electrodynamics is, along with general relativity
General relativity, also known as the general theory of relativity, and as Einstein's theory of gravity, is the differential geometry, geometric theory of gravitation published by Albert Einstein in 1915 and is the current description of grav ...
, one of the most accurate physical theories ever devised.
The full apparatus of quantum field theory is often unnecessary for describing electrodynamic systems. A simpler approach, one that has been used since the inception of quantum mechanics, is to treat charged particles as quantum mechanical objects being acted on by a classical electromagnetic field
An electromagnetic field (also EM field) is a physical field, varying in space and time, that represents the electric and magnetic influences generated by and acting upon electric charges. The field at any point in space and time can be regarde ...
. For example, the elementary quantum model of the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb for ...
describes the electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
of the hydrogen atom using a classical Coulomb potential. Likewise, in a Stern–Gerlach experiment, a charged particle is modeled as a quantum system, while the background magnetic field is described classically. This "semi-classical" approach fails if quantum fluctuations in the electromagnetic field play an important role, such as in the emission of photons by charged particle
In physics, a charged particle is a particle with an electric charge. For example, some elementary particles, like the electron or quarks are charged. Some composite particles like protons are charged particles. An ion, such as a molecule or atom ...
s.
Quantum field theories for the strong nuclear force and the weak nuclear force have also been developed. The quantum field theory of the strong nuclear force is called quantum chromodynamics
In theoretical physics, quantum chromodynamics (QCD) is the study of the strong interaction between quarks mediated by gluons. Quarks are fundamental particles that make up composite hadrons such as the proton, neutron and pion. QCD is a type of ...
, and describes the interactions of subnuclear particles such as quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nucleus, atomic nuclei ...
s and gluon
A gluon ( ) is a type of Massless particle, massless elementary particle that mediates the strong interaction between quarks, acting as the exchange particle for the interaction. Gluons are massless vector bosons, thereby having a Spin (physi ...
s. The weak nuclear force and the electromagnetic force were unified, in their quantized forms, into a single quantum field theory (known as electroweak theory
In particle physics, the electroweak interaction or electroweak force is the unified description of two of the fundamental interactions of nature: electromagnetism (electromagnetic interaction) and the weak interaction. Although these two forc ...
), by the physicists Abdus Salam, Sheldon Glashow and Steven Weinberg
Steven Weinberg (; May 3, 1933 – July 23, 2021) was an American theoretical physicist and Nobel laureate in physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic inter ...
.
Relation to general relativity
Even though the predictions of both quantum theory and general relativity have been supported by rigorous and repeatedempirical evidence
Empirical evidence is evidence obtained through sense experience or experimental procedure. It is of central importance to the sciences and plays a role in various other fields, like epistemology and law.
There is no general agreement on how the ...
, their abstract formalisms contradict each other and they have proven extremely difficult to incorporate into one consistent, cohesive model. Gravity is negligible in many areas of particle physics, so that unification between general relativity and quantum mechanics is not an urgent issue in those particular applications. However, the lack of a correct theory of quantum gravity
Quantum gravity (QG) is a field of theoretical physics that seeks to describe gravity according to the principles of quantum mechanics. It deals with environments in which neither gravitational nor quantum effects can be ignored, such as in the v ...
is an important issue in physical cosmology
Physical cosmology is a branch of cosmology concerned with the study of cosmological models. A cosmological model, or simply cosmology, provides a description of the largest-scale structures and dynamics of the universe and allows study of fu ...
and the search by physicists for an elegant "Theory of Everything
A theory of everything (TOE), final theory, ultimate theory, unified field theory, or master theory is a hypothetical singular, all-encompassing, coherent theoretical physics, theoretical framework of physics that fully explains and links togeth ...
" (TOE). Consequently, resolving the inconsistencies between both theories has been a major goal of 20th- and 21st-century physics. This TOE would combine not only the models of subatomic physics but also derive the four fundamental forces of nature from a single force or phenomenon.
One proposal for doing so is string theory
In physics, string theory is a theoretical framework in which the point-like particles of particle physics are replaced by one-dimensional objects called strings. String theory describes how these strings propagate through space and intera ...
, which posits that the point-like particles of particle physics
Particle physics or high-energy physics is the study of Elementary particle, fundamental particles and fundamental interaction, forces that constitute matter and radiation. The field also studies combinations of elementary particles up to the s ...
are replaced by one-dimensional objects called strings. String theory describes how these strings propagate through space and interact with each other. On distance scales larger than the string scale, a string looks just like an ordinary particle, with its mass
Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ...
, charge, and other properties determined by the vibration
Vibration () is a mechanical phenomenon whereby oscillations occur about an equilibrium point. Vibration may be deterministic if the oscillations can be characterised precisely (e.g. the periodic motion of a pendulum), or random if the os ...
al state of the string. In string theory, one of the many vibrational states of the string corresponds to the graviton
In theories of quantum gravity, the graviton is the hypothetical elementary particle that mediates the force of gravitational interaction. There is no complete quantum field theory of gravitons due to an outstanding mathematical problem with re ...
, a quantum mechanical particle that carries gravitational force.
Another popular theory is loop quantum gravity
Loop quantum gravity (LQG) is a theory of quantum gravity that incorporates matter of the Standard Model into the framework established for the intrinsic quantum gravity case. It is an attempt to develop a quantum theory of gravity based direc ...
(LQG), which describes quantum properties of gravity and is thus a theory of quantum spacetime. LQG is an attempt to merge and adapt standard quantum mechanics and standard general relativity. This theory describes space as an extremely fine fabric "woven" of finite loops called spin network
In physics, a spin network is a type of diagram which can be used to represent states and interactions between particles and fields in quantum mechanics. From a mathematical perspective, the diagrams are a concise way to represent multilinear ...
s. The evolution of a spin network over time is called a spin foam. The characteristic length scale of a spin foam is the Planck length, approximately 1.616×10−35 m, and so lengths shorter than the Planck length are not physically meaningful in LQG.
Philosophical implications
Since its inception, the many counter-intuitive aspects and results of quantum mechanics have provoked strongphilosophical
Philosophy ('love of wisdom' in Ancient Greek) is a systematic study of general and fundamental questions concerning topics like existence, reason, knowledge, Value (ethics and social sciences), value, mind, and language. It is a rational an ...
debates and many interpretations. The arguments centre on the probabilistic nature of quantum mechanics, the difficulties with wavefunction collapse
In various interpretations of quantum mechanics, wave function collapse, also called reduction of the state vector, occurs when a wave function—initially in a superposition of several eigenstates—reduces to a single eigenstate due to i ...
and the related measurement problem
In quantum mechanics, the measurement problem is the ''problem of definite outcomes:'' quantum systems have superpositions but quantum measurements only give one definite result.
The wave function in quantum mechanics evolves deterministically ...
, and quantum nonlocality. Perhaps the only consensus that exists about these issues is that there is no consensus. Richard Feynman
Richard Phillips Feynman (; May 11, 1918 – February 15, 1988) was an American theoretical physicist. He is best known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, the physics of t ...
once said, "I think I can safely say that nobody understands quantum mechanics." According to Steven Weinberg
Steven Weinberg (; May 3, 1933 – July 23, 2021) was an American theoretical physicist and Nobel laureate in physics for his contributions with Abdus Salam and Sheldon Glashow to the unification of the weak force and electromagnetic inter ...
, "There is now in my opinion no entirely satisfactory interpretation of quantum mechanics."
The views of Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Werner Heisenberg and other physicists are often grouped together as the "Copenhagen interpretation
The Copenhagen interpretation is a collection of views about the meaning of quantum mechanics, stemming from the work of Niels Bohr, Werner Heisenberg, Max Born, and others. While "Copenhagen" refers to the Danish city, the use as an "interpretat ...
". According to these views, the probabilistic nature of quantum mechanics is not a temporary feature which will eventually be replaced by a deterministic theory, but is instead a final renunciation of the classical idea of "causality". Bohr in particular emphasized that any well-defined application of the quantum mechanical formalism must always make reference to the experimental arrangement, due to the complementary nature of evidence obtained under different experimental situations. Copenhagen-type interpretations were adopted by Nobel laureates in quantum physics, including Bohr, Heisenberg, Schrödinger, Feynman, and Zeilinger as well as 21st-century researchers in quantum foundations.
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence f ...
, himself one of the founders of quantum theory, was troubled by its apparent failure to respect some cherished metaphysical principles, such as determinism
Determinism is the Metaphysics, metaphysical view that all events within the universe (or multiverse) can occur only in one possible way. Deterministic theories throughout the history of philosophy have developed from diverse and sometimes ov ...
and locality. Einstein's long-running exchanges with Bohr about the meaning and status of quantum mechanics are now known as the Bohr–Einstein debates. Einstein believed that underlying quantum mechanics must be a theory that explicitly forbids action at a distance
Action at a distance is the concept in physics that an object's motion (physics), motion can be affected by another object without the two being in Contact mechanics, physical contact; that is, it is the concept of the non-local interaction of ob ...
. He argued that quantum mechanics was incomplete, a theory that was valid but not fundamental, analogous to how thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
is valid, but the fundamental theory behind it is statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applicati ...
. In 1935, Einstein and his collaborators Boris Podolsky and Nathan Rosen published an argument that the principle of locality implies the incompleteness of quantum mechanics, a thought experiment
A thought experiment is an imaginary scenario that is meant to elucidate or test an argument or theory. It is often an experiment that would be hard, impossible, or unethical to actually perform. It can also be an abstract hypothetical that is ...
later termed the Einstein–Podolsky–Rosen paradox. In 1964, John Bell showed that EPR's principle of locality, together with determinism, was actually incompatible with quantum mechanics: they implied constraints on the correlations produced by distance systems, now known as Bell inequalities, that can be violated by entangled particles. Since then several experiments have been performed to obtain these correlations, with the result that they do in fact violate Bell inequalities, and thus falsify the conjunction of locality with determinism.
Bohmian mechanics shows that it is possible to reformulate quantum mechanics to make it deterministic, at the price of making it explicitly nonlocal. It attributes not only a wave function to a physical system, but in addition a real position, that evolves deterministically under a nonlocal guiding equation. The evolution of a physical system is given at all times by the Schrödinger equation together with the guiding equation; there is never a collapse of the wave function. This solves the measurement problem.
many-worlds interpretation
The many-worlds interpretation (MWI) is an interpretation of quantum mechanics that asserts that the universal wavefunction is Philosophical realism, objectively real, and that there is no wave function collapse. This implies that all Possible ...
, formulated in 1956, holds that all the possibilities described by quantum theory simultaneously occur in a multiverse composed of mostly independent parallel universes. This is a consequence of removing the axiom of the collapse of the wave packet. All possible states of the measured system and the measuring apparatus, together with the observer, are present in a real physical quantum superposition. While the multiverse is deterministic, we perceive non-deterministic behavior governed by probabilities, because we do not observe the multiverse as a whole, but only one parallel universe at a time. Exactly how this is supposed to work has been the subject of much debate. Several attempts have been made to make sense of this and derive the Born rule, with no consensus on whether they have been successful.
Relational quantum mechanics appeared in the late 1990s as a modern derivative of Copenhagen-type ideas, and QBism was developed some years later.
History
Quantum mechanics was developed in the early decades of the 20th century, driven by the need to explain phenomena that, in some cases, had been observed in earlier times. Scientific inquiry into the wave nature of light began in the 17th and 18th centuries, when scientists such asRobert Hooke
Robert Hooke (; 18 July 16353 March 1703) was an English polymath who was active as a physicist ("natural philosopher"), astronomer, geologist, meteorologist, and architect. He is credited as one of the first scientists to investigate living ...
, Christiaan Huygens
Christiaan Huygens, Halen, Lord of Zeelhem, ( , ; ; also spelled Huyghens; ; 14 April 1629 – 8 July 1695) was a Dutch mathematician, physicist, engineer, astronomer, and inventor who is regarded as a key figure in the Scientific Revolution ...
and Leonhard Euler
Leonhard Euler ( ; ; ; 15 April 170718 September 1783) was a Swiss polymath who was active as a mathematician, physicist, astronomer, logician, geographer, and engineer. He founded the studies of graph theory and topology and made influential ...
proposed a wave theory of light based on experimental observations. In 1803 English polymath
A polymath or polyhistor is an individual whose knowledge spans many different subjects, known to draw on complex bodies of knowledge to solve specific problems. Polymaths often prefer a specific context in which to explain their knowledge, ...
Thomas Young described the famous double-slit experiment
In modern physics, the double-slit experiment demonstrates that light and matter can exhibit behavior of both classical particles and classical waves. This type of experiment was first performed by Thomas Young in 1801, as a demonstration of ...
. This experiment played a major role in the general acceptance of the wave theory of light
In physics, physical optics, or wave optics, is the branch of optics that studies interference, diffraction, polarization, and other phenomena for which the ray approximation of geometric optics is not valid. This usage tends not to include effec ...
.
During the early 19th century, chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
research by John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He introduced the atomic theory into chemistry. He also researched Color blindness, colour blindness; as a result, the umbrella term ...
and Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (, also , ; 9 August 17769 July 1856) was an Italian scientist, most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volu ...
lent weight to the atomic theory
Atomic theory is the scientific theory that matter is composed of particles called atoms. The definition of the word "atom" has changed over the years in response to scientific discoveries. Initially, it referred to a hypothetical concept of ...
of matter, an idea that James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish physicist and mathematician who was responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism an ...
, Ludwig Boltzmann
Ludwig Eduard Boltzmann ( ; ; 20 February 1844 – 5 September 1906) was an Austrian mathematician and Theoretical physics, theoretical physicist. His greatest achievements were the development of statistical mechanics and the statistical ex ...
and others built upon to establish the kinetic theory of gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small ...
. The successes of kinetic theory gave further credence to the idea that matter is composed of atoms, yet the theory also had shortcomings that would only be resolved by the development of quantum mechanics. Reprinted, Addison-Wesley, 1989, While the early conception of atoms from Greek philosophy
Ancient Greek philosophy arose in the 6th century BC. Philosophy was used to make sense of the world using reason. It dealt with a wide variety of subjects, including astronomy, epistemology, mathematics, political philosophy, ethics, metaphysic ...
had been that they were indivisible unitsthe word "atom" deriving from the Greek
Greek may refer to:
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group
*Greek language, a branch of the Indo-European language family
**Proto-Greek language, the assumed last common ancestor of all kno ...
for 'uncuttable'the 19th century saw the formulation of hypotheses about subatomic structure. One important discovery in that regard was Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
's 1838 observation of a glow caused by an electrical discharge inside a glass tube containing gas at low pressure. Julius Plücker
Julius Plücker (16 June 1801 – 22 May 1868) was a German mathematician and physicist. He made fundamental contributions to the field of analytical geometry and was a pioneer in the investigations of cathode rays that led eventually to the di ...
, Johann Wilhelm Hittorf and Eugen Goldstein carried on and improved upon Faraday's work, leading to the identification of cathode rays
Cathode rays are streams of electrons observed in vacuum tube, discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitte ...
, which J. J. Thomson found to consist of subatomic particles that would be called electrons.
The black-body radiation
Black-body radiation is the thermal radiation, thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific ...
problem was discovered by Gustav Kirchhoff
Gustav Robert Kirchhoff (; 12 March 1824 – 17 October 1887) was a German chemist, mathematician, physicist, and spectroscopist who contributed to the fundamental understanding of electrical circuits, spectroscopy and the emission of black-body ...
in 1859. In 1900, Max Planck proposed the hypothesis that energy is radiated and absorbed in discrete "quanta" (or energy packets), yielding a calculation that precisely matched the observed patterns of black-body radiation. The word ''quantum'' derives from the Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
, meaning "how great" or "how much". According to Planck, quantities of energy could be thought of as divided into "elements" whose size (''E'') would be proportional to their frequency
Frequency is the number of occurrences of a repeating event per unit of time. Frequency is an important parameter used in science and engineering to specify the rate of oscillatory and vibratory phenomena, such as mechanical vibrations, audio ...
(''ν''):
,
where ''h'' is the Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
. Planck cautiously insisted that this was only an aspect of the processes of absorption and emission of radiation and was not the physical reality of the radiation. In fact, he considered his quantum hypothesis a mathematical trick to get the right answer rather than a sizable discovery. However, in 1905 Albert Einstein interpreted Planck's quantum hypothesis realistically and used it to explain the photoelectric effect
The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physi ...
, in which shining light on certain materials can eject electrons from the material. Niels Bohr then developed Planck's ideas about radiation into a model of the hydrogen atom that successfully predicted the spectral line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency ...
s of hydrogen. Einstein further developed this idea to show that an electromagnetic wave
In physics, electromagnetic radiation (EMR) is a self-propagating wave of the electromagnetic field that carries momentum and radiant energy through space. It encompasses a broad spectrum, classified by frequency or its inverse, wavelength, ...
such as light could also be described as a particle (later called the photon), with a discrete amount of energy that depends on its frequency. In his paper "On the Quantum Theory of Radiation", Einstein expanded on the interaction between energy and matter to explain the absorption and emission of energy by atoms. Although overshadowed at the time by his general theory of relativity, this paper articulated the mechanism underlying the stimulated emission of radiation, which became the basis of the laser.
 This phase is known as the
This phase is known as the old quantum theory
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The th ...
. Never complete or self-consistent, the old quantum theory was rather a set of heuristic
A heuristic or heuristic technique (''problem solving'', '' mental shortcut'', ''rule of thumb'') is any approach to problem solving that employs a pragmatic method that is not fully optimized, perfected, or rationalized, but is nevertheless ...
corrections to classical mechanics. The theory is now understood as a semi-classical approximation to modern quantum mechanics. Notable results from this period include, in addition to the work of Planck, Einstein and Bohr mentioned above, Einstein and Peter Debye
Peter Joseph William Debye ( ; born Petrus Josephus Wilhelmus Debije, ; March 24, 1884 – November 2, 1966) was a Dutch-American physicist and physical chemist, and Nobel laureate in Chemistry.
Biography
Early life
Born in Maastricht, Neth ...
's work on the specific heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat ...
of solids, Bohr and Hendrika Johanna van Leeuwen
Hendrika Johanna van Leeuwen (July 3, 1887 – February 26, 1974) was a Dutch physicist known for her early contributions to the theory of magnetism. She studied at Leiden University under the guidance of Hendrik Antoon Lorentz, obtaining her do ...
's proof
Proof most often refers to:
* Proof (truth), argument or sufficient evidence for the truth of a proposition
* Alcohol proof, a measure of an alcoholic drink's strength
Proof may also refer to:
Mathematics and formal logic
* Formal proof, a co ...
that classical physics cannot account for diamagnetism
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnet ...
, and Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and ...
's extension of the Bohr model to include special-relativistic effects.
In the mid-1920s quantum mechanics was developed to become the standard formulation for atomic physics. In 1923, the French physicist Louis de Broglie put forward his theory of matter waves by stating that particles can exhibit wave characteristics and vice versa. Building on de Broglie's approach, modern quantum mechanics was born in 1925, when the German physicists Werner Heisenberg, Max Born, and Pascual Jordan
Ernst Pascual Jordan (; 18 October 1902 – 31 July 1980) was a German theoretical and mathematical physicist who made significant contributions to quantum mechanics and quantum field theory. He contributed much to the mathematical form of matri ...
David Edwards, "The Mathematical Foundations of Quantum Mechanics", ''Synthese'', Volume 42, Number 1/September, 1979, pp. 1–70.David Edwards, "The Mathematical Foundations of Quantum Field Theory: Fermions, Gauge Fields, and Super-symmetry, Part I: Lattice Field Theories", ''International Journal of Theoretical Physics'', Vol. 20, No. 7 (1981). developed matrix mechanics and the Austrian physicist Erwin Schrödinger invented wave mechanics. Born introduced the probabilistic interpretation of Schrödinger's wave function in July 1926. Thus, the entire field of quantum physics emerged, leading to its wider acceptance at the Fifth Solvay Conference in 1927.
By 1930, quantum mechanics had been further unified and formalized by David Hilbert
David Hilbert (; ; 23 January 1862 – 14 February 1943) was a German mathematician and philosopher of mathematics and one of the most influential mathematicians of his time.
Hilbert discovered and developed a broad range of fundamental idea ...
, Paul Dirac and John von Neumann
John von Neumann ( ; ; December 28, 1903 – February 8, 1957) was a Hungarian and American mathematician, physicist, computer scientist and engineer. Von Neumann had perhaps the widest coverage of any mathematician of his time, in ...
with greater emphasis on measurement
Measurement is the quantification of attributes of an object or event, which can be used to compare with other objects or events.
In other words, measurement is a process of determining how large or small a physical quantity is as compared to ...
, the statistical nature of our knowledge of reality, and philosophical speculation about the 'observer'. It has since permeated many disciplines, including quantum chemistry, quantum electronics
Quantum optics is a branch of atomic, molecular, and optical physics and quantum chemistry that studies the behavior of photons (individual quanta of light). It includes the study of the particle-like properties of photons and their interaction w ...
, quantum optics
Quantum optics is a branch of atomic, molecular, and optical physics and quantum chemistry that studies the behavior of photons (individual quanta of light). It includes the study of the particle-like properties of photons and their interaction ...
, and quantum information science
Quantum information science is a field that combines the principles of quantum mechanics with information theory to study the processing, analysis, and transmission of information. It covers both theoretical and experimental aspects of quantum phys ...
. It also provides a useful framework for many features of the modern periodic table of elements, and describes the behaviors of atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
during chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
ing and the flow of electrons in computer semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s, and therefore plays a crucial role in many modern technologies. While quantum mechanics was constructed to describe the world of the very small, it is also needed to explain some macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenome ...
phenomena such as superconductors
Superconductivity is a set of physical properties observed in superconductors: materials where electrical resistance vanishes and magnetic fields are expelled from the material. Unlike an ordinary metallic conductor, whose resistance decreases ...
and superfluid
Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortex, vortices that continue to rotate indefinitely. Superfluidity occurs ...
s.
See also
*Bra–ket notation
Bra–ket notation, also called Dirac notation, is a notation for linear algebra and linear operators on complex vector spaces together with their dual space both in the finite-dimensional and infinite-dimensional case. It is specifically de ...
* Einstein's thought experiments
* List of textbooks on classical mechanics and quantum mechanics
* Macroscopic quantum phenomena
Macroscopic quantum phenomena are processes showing Quantum mechanics, quantum behavior at the macroscopic scale, rather than at the Atom, atomic scale where quantum effects are prevalent. The best-known examples of macroscopic quantum phenomena ar ...
* Phase-space formulation
The phase space of a physical system is the set of all possible physical states of the system when described by a given parameterization. Each possible state corresponds uniquely to a point in the phase space. For mechanical systems, the p ...
* Regularization (physics)
In physics, especially quantum field theory, regularization is a method of modifying observables which have singularities in order to make them finite by the introduction of a suitable parameter called the regulator. The regulator, also known ...
* Two-state quantum system
In quantum mechanics, a two-state system (also known as a two-level system) is a quantum system that can exist in any quantum superposition of two independent (physically distinguishable) quantum states. The Hilbert space describing such a syste ...
Explanatory notes
References
Further reading
The following titles, all by working physicists, attempt to communicate quantum theory to lay people, using a minimum of technical apparatus: * Chester, Marvin (1987). ''Primer of Quantum Mechanics''. John Wiley. * *Richard Feynman
Richard Phillips Feynman (; May 11, 1918 – February 15, 1988) was an American theoretical physicist. He is best known for his work in the path integral formulation of quantum mechanics, the theory of quantum electrodynamics, the physics of t ...
, 1985. '' QED: The Strange Theory of Light and Matter'', Princeton University Press. . Four elementary lectures on quantum electrodynamics and quantum field theory
In theoretical physics, quantum field theory (QFT) is a theoretical framework that combines Field theory (physics), field theory and the principle of relativity with ideas behind quantum mechanics. QFT is used in particle physics to construct phy ...
, yet containing many insights for the expert.
* Ghirardi, GianCarlo, 2004. ''Sneaking a Look at God's Cards'', Gerald Malsbary, trans. Princeton Univ. Press. The most technical of the works cited here. Passages using algebra
Algebra is a branch of mathematics that deals with abstract systems, known as algebraic structures, and the manipulation of expressions within those systems. It is a generalization of arithmetic that introduces variables and algebraic ope ...
, trigonometry
Trigonometry () is a branch of mathematics concerned with relationships between angles and side lengths of triangles. In particular, the trigonometric functions relate the angles of a right triangle with ratios of its side lengths. The fiel ...
, and bra–ket notation
Bra–ket notation, also called Dirac notation, is a notation for linear algebra and linear operators on complex vector spaces together with their dual space both in the finite-dimensional and infinite-dimensional case. It is specifically de ...
can be passed over on a first reading.
* N. David Mermin, 1990, "Spooky actions at a distance: mysteries of the QT" in his ''Boojums All the Way Through''. Cambridge University Press: 110–76.
* Victor Stenger, 2000. ''Timeless Reality: Symmetry, Simplicity, and Multiple Universes''. Buffalo, New York: Prometheus Books. Chpts. 5–8. Includes cosmological and philosophical considerations.
More technical:
*
*
*
*
* Bryce DeWitt, R. Neill Graham, eds., 1973. ''The Many-Worlds Interpretation of Quantum Mechanics'', Princeton Series in Physics, Princeton University Press.
*
*
* D. Greenberger, K. Hentschel, F. Weinert, eds., 2009. ''Compendium of quantum physics, Concepts, experiments, history and philosophy'', Springer-Verlag, Berlin, Heidelberg. Short articles on many QM topics.
* A standard undergraduate text.
* Max Jammer
Max Jammer (; born Moshe Jammer, ; 13 April 1915 – 18 December 2010), was an Israeli physicist and philosophy of physics, philosopher of physics. He was born in Berlin, Germany. He was Rector and Acting President at Bar-Ilan University from 19 ...
, 1966. ''The Conceptual Development of Quantum Mechanics''. McGraw Hill.
* Hagen Kleinert, 2004. ''Path Integrals in Quantum Mechanics, Statistics, Polymer Physics, and Financial Markets'', 3rd ed. Singapore: World ScientificDraft of 4th edition.
*
Online copy
* * Gunther Ludwig, 1968. ''Wave Mechanics''. London: Pergamon Press. * George Mackey (2004). ''The mathematical foundations of quantum mechanics''. Dover Publications. . * * Albert Messiah, 1966. ''Quantum Mechanics'' (Vol. I), English translation from French by G. M. Temmer. North Holland, John Wiley & Sons. Cf. chpt. IV, section III
online
* * Considers the extent to which chemistry and the periodic system have been reduced to quantum mechanics. * * * * * Veltman, Martinus J. G. (2003), ''Facts and Mysteries in Elementary Particle Physics''.
External links
Introduction to Quantum Theory at Quantiki.
Quantum Physics Made Relatively Simple
three video lectures by Hans Bethe. Course material
Quantum Cook Book
an
PHYS 201: Fundamentals of Physics II
by Ramamurti Shankar, Yale OpenCourseware. *
Modern Physics: With waves, thermodynamics, and optics
' – an online textbook. *
MIT OpenCourseWare
MIT OpenCourseWare (MIT OCW) is an initiative of the Massachusetts Institute of Technology (MIT) to publish all of the educational materials from its undergraduate- and graduate-level courses online, freely and openly available to anyone, anywh ...
Chemistry
an
Physics
Se
8.04
an
Examples in Quantum Mechanics
Philosophy * * {{Authority control