|
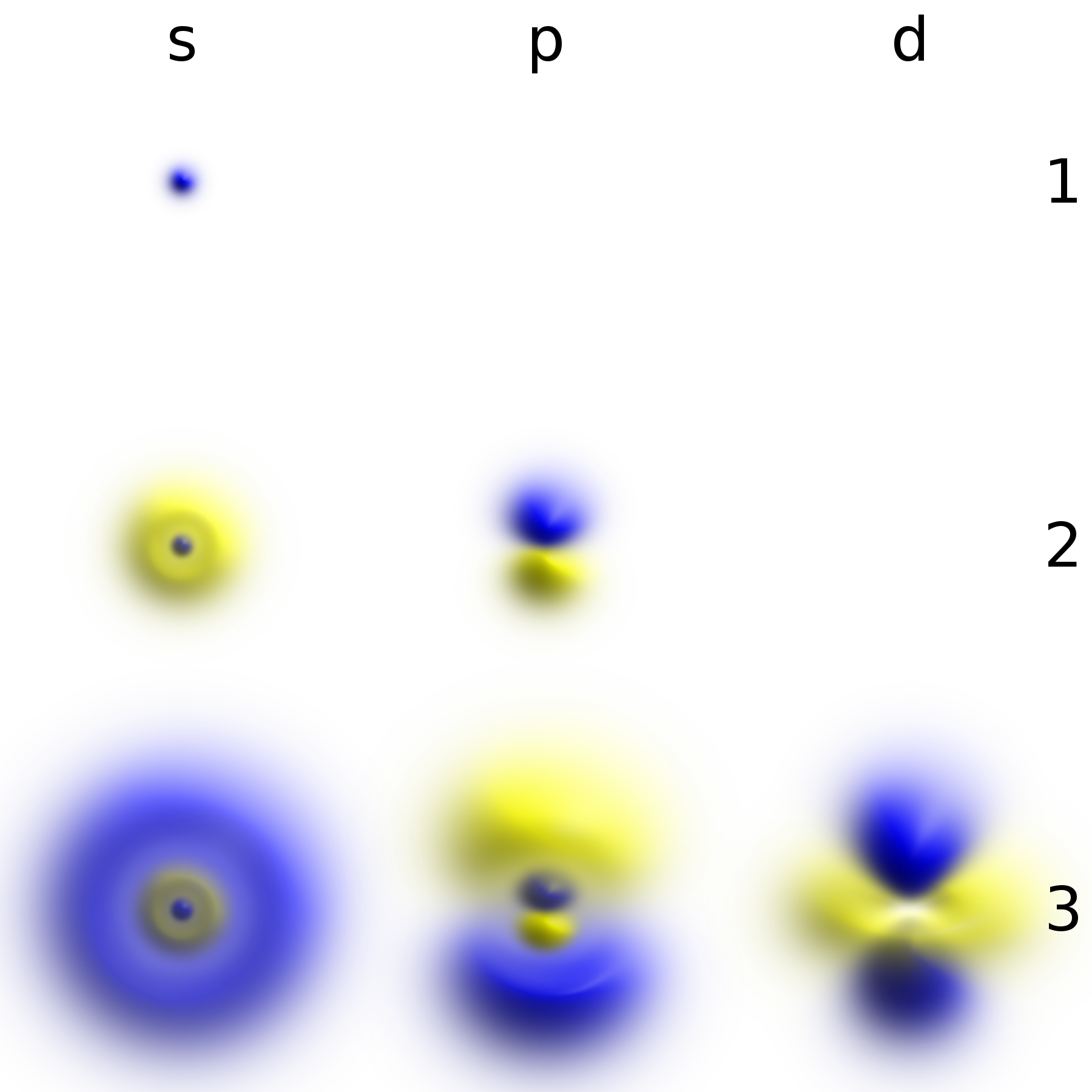
List Of Quantum-mechanical Systems With Analytical Solutions
Much insight in quantum mechanics can be gained from understanding the closed-form solutions to the time-dependent non-relativistic Schrödinger equation. It takes the form \hat \psi = \left - \frac \nabla^2 + V \right\psi = i\hbar \frac, where \psi is the wave function of the system, \hat is the Hamiltonian operator, and t is time. Stationary states of this equation are found by solving the time-independent Schrödinger equation, \left - \frac \nabla^2 + V \right\psi = E \psi , which is an eigenvalue equation. Very often, only numerical solutions to the Schrödinger equation can be found for a given physical system and its associated potential energy. However, there exists a subset of physical systems for which the form of the eigenfunctions and their associated energies, or eigenvalues, can be found. These quantum-mechanical systems with analytical solutions are listed below. Solvable systems *The two-state quantum system (the simplest possible quantum system) *The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Harmonic Oscillator
The quantum harmonic oscillator is the quantum-mechanical analog of the classical harmonic oscillator. Because an arbitrary smooth potential can usually be approximated as a harmonic potential at the vicinity of a stable equilibrium point, it is one of the most important model systems in quantum mechanics. Furthermore, it is one of the few quantum-mechanical systems for which an exact, analytical solution is known. One-dimensional harmonic oscillator Hamiltonian and energy eigenstates The Hamiltonian of the particle is: \hat H = \frac + \frac k ^2 = \frac + \frac m \omega^2 ^2 \, , where is the particle's mass, is the force constant, \omega = \sqrt is the angular frequency of the oscillator, \hat is the position operator (given by in the coordinate basis), and \hat is the momentum operator (given by \hat p = -i \hbar \, \partial / \partial x in the coordinate basis). The first term in the Hamiltonian represents the kinetic energy of the particle, and the second ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science. Quantum mechanics can describe many systems that classical physics cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic and Microscopic scale, (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales. Quantum systems have Bound state, bound states that are Quantization (physics), quantized to Discrete mathematics, discrete values of energy, momentum, angular momentum, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Airy Function
In the physical sciences, the Airy function (or Airy function of the first kind) is a special function named after the British astronomer George Biddell Airy (1801–1892). The function Ai(''x'') and the related function Bi(''x''), are Linear independence, linearly independent solutions to the differential equation \frac - xy = 0 , known as the Airy equation or the Stokes equation. Because the solution of the linear differential equation \frac - ky = 0 is oscillatory for and exponential for , the Airy functions are oscillatory for and exponential for . In fact, the Airy equation is the simplest second-order linear differential equation with a turning point (a point where the character of the solutions changes from oscillatory to exponential). Definitions For real values of , the Airy function of the first kind can be defined by the improper integral, improper Riemann integral: \operatorname(x) = \dfrac\int_0^\infty\cos\left(\dfrac + xt\right)\, dt\equiv \dfrac \lim_ \in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirichlet Boundary Condition
In mathematics, the Dirichlet boundary condition is imposed on an ordinary or partial differential equation, such that the values that the solution takes along the boundary of the domain are fixed. The question of finding solutions to such equations is known as the Dirichlet problem. In the sciences and engineering, a Dirichlet boundary condition may also be referred to as a fixed boundary condition or boundary condition of the first type. It is named after Peter Gustav Lejeune Dirichlet (1805–1859). In finite-element analysis, the ''essential'' or Dirichlet boundary condition is defined by weighted-integral form of a differential equation. The dependent unknown ''u in the same form as the weight function w'' appearing in the boundary expression is termed a ''primary variable'', and its specification constitutes the ''essential'' or Dirichlet boundary condition. Examples ODE For an ordinary differential equation, for instance, y'' + y = 0, the Dirichlet boundary conditions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positronium
Positronium (Ps) is a system consisting of an electron and its antimatter, anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annihilate each other to predominantly produce two or three gamma-rays, depending on the relative spin states. The energy levels of the two particles are similar to that of the hydrogen atom (which is a bound state of a proton and an electron). However, because of the reduced mass, the frequency, frequencies of the spectral lines are less than half of those for the corresponding hydrogen lines. States The mass of positronium is 1.022 MeV, which is twice the electron mass minus the binding energy of a few eV. The lowest energy orbital state of positronium is 1S, and like with hydrogen, it has a hyperfine structure arising from the relative orientations of the spins of the electron and the positron. The Singlet state, ''singlet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen-like Atom
A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like atoms include, but are not limited to, hydrogen itself, all alkali metals such as Rb and Cs, singly ionized alkaline earth metals such as Ca+ and Sr+ and other ions such as He+, Li2+, and Be3+ and isotopes of any of the above. A hydrogen-like atom includes a positively charged core consisting of the atomic nucleus and any core electrons as well as a single valence electron. Because helium is common in the universe, the spectroscopy of singly ionized helium is important in EUV astronomy, for example, of DO white dwarf stars. The non-relativistic Schrödinger equation and relativistic Dirac equation for the hydrogen atom can be solved analytically, owing to the simplicity of the two-particle physical system. The one-electron wave function solutions are referred to as ''hydrogen-like atomic orbitals''. Hydrogen-li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle In A Spherically Symmetric Potential
In quantum mechanics, a spherically symmetric potential is a system of which the potential only depends on the radial distance from the spherical center and a location in space. A particle in a spherically symmetric potential will behave accordingly to said potential and can therefore be used as an approximation, for example, of the electron in a hydrogen atom or of the formation of chemical bonds. In the general time-independent case, the dynamics of a particle in a spherically symmetric potential are governed by a Hamiltonian of the following form:\hat = \frac + V() Here, m_0 is the mass of the particle, \hat is the momentum operator, and the potential V(r) depends only on the vector magnitude of the position vector, that is, the radial distance from the origin (hence the spherical symmetry of the problem). To describe a particle in a spherically symmetric system, it is convenient to use spherical coordinates; denoted by r, \theta and \phi. The time-independent Schrödin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rigid Rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special rigid rotor is the ''linear rotor'' requiring only two angles to describe, for example of a diatomic molecule. More general molecules are 3-dimensional, such as water (asymmetric rotor), ammonia (symmetric rotor), or methane (spherical rotor). Linear rotor The linear rigid rotor model consists of two point masses located at fixed distances from their center of mass. The fixed distance between the two masses and the values of the masses are the only characteristics of the rigid model. However, for many actual diatomics this model is too restrictive since distances are usually not completely fixed. Corrections on the rigid model can be made to compensate for small variations in the distance. Even in such a case the rigid rotor model is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Pendulum
The quantum pendulum is fundamental in understanding hindered internal rotations in chemistry, quantum features of scattering atoms, as well as numerous other quantum phenomena. Though a pendulum not subject to the small-angle approximation has an inherent nonlinearity, the Schrödinger equation for the quantized system can be solved relatively easily. Schrödinger equation Using Lagrangian mechanics from classical mechanics, one can develop a Hamiltonian (quantum mechanics), Hamiltonian for the system. A simple pendulum has one generalized coordinate (the angular displacement \phi) and two constraints (the length of the string and the plane of motion). The kinetic and potential energies of the system can be found to be :T = \frac m l^2 \dot^2, :U = mgl (1 - \cos\phi). This results in the Hamiltonian :\hat = \frac + mgl (1 - \cos\phi). The time-dependent Schrödinger equation for the system is :i \hbar \frac = -\frac \frac + mgl (1 - \cos\phi) \Psi. One must solve the time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pöschl–Teller Potential
In mathematical physics, a Pöschl–Teller potential, named after the physicists Herta Pöschl (credited as G. Pöschl) and Edward Teller, is a special class of potentials for which the one-dimensional Schrödinger equation can be solved in terms of special functions Special functions are particular mathematical functions that have more or less established names and notations due to their importance in mathematical analysis, functional analysis, geometry, physics, or other applications. The term is defined by .... Definition In its symmetric form is explicitly given by : V(x) =-\frac\mathrm^2(x) and the solutions of the time-independent Schrödinger equation : -\frac\psi''(x)+ V(x)\psi(x)=E\psi(x) with this potential can be found by virtue of the substitution u=\mathrm, which yields : \left 1-u^2)\psi'(u)\right+\lambda(\lambda+1)\psi(u)+\frac\psi(u)=0 . Thus the solutions \psi(u) are just the Legendre functions P_\lambda^\mu(\tanh(x)) with E=-\frac, and \lambda=1, 2, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |