neptunium on:
[Wikipedia]
[Google]
[Amazon]
Neptunium is a
Rn">Radon.html" ;"title="/nowiki>Radon">Rn/nowiki> 5f4 6d1 7s2. This differs from the configuration expected by the Aufbau principle in that one electron is in the 6d Electron shell#Subshells, subshell instead of being as expected in the 5f subshell. This is because of the similarity of the electron energies of the 5f, 6d, and 7s subshells. In forming compounds and ions, all the valence electrons may be lost, leaving behind an inert core of inner electrons with the electron configuration of the
 When the first
When the first
 As research on nuclear fission progressed in early 1939, Edwin McMillan at the Berkeley Radiation Laboratory of the University of California, Berkeley decided to run an experiment bombarding uranium using the powerful 60-inch (1.52 m) cyclotron that had recently been built at the university. The purpose was to separate the various fission products produced by the bombardment by exploiting the enormous force that the fragments gain from their mutual electrical repulsion after fissioning. Although he did not discover anything of note from this, McMillan did observe two new beta decay half-lives in the uranium trioxide target itself, which meant that whatever was producing the radioactivity had not violently repelled each other like normal fission products. He quickly realized that one of the half-lives closely matched the known 23-minute decay period of uranium-239, but the other half-life of 2.3 days was unknown. McMillan took the results of his experiment to chemist and fellow Berkeley professor Emilio Segrè to attempt to isolate the source of the radioactivity. Both scientists began their work using the prevailing theory that element 93 would have similar chemistry to rhenium, but Segrè rapidly determined that McMillan's sample was not at all similar to rhenium. Instead, when he reacted it with hydrogen fluoride (HF) with a strong oxidizing agent present, it behaved much like members of the rare earths. Since these elements comprise a large percentage of fission products, Segrè and McMillan decided that the half-life must have been simply another fission product, titling the paper "An Unsuccessful Search for Transuranium Elements".
However, as more information about fission became available, the possibility that the fragments of nuclear fission could still have been present in the target became more remote. McMillan and several scientists, including Philip H. Abelson, attempted again to determine what was producing the unknown half-life. In early 1940, McMillan realized that his 1939 experiment with Segrè had failed to test the chemical reactions of the radioactive source with sufficient rigor. In a new experiment, McMillan tried subjecting the unknown substance to HF in the presence of a reducing agent, something he had not done before. This reaction resulted in the sample Precipitation (chemistry), precipitating with the HF, an action that definitively ruled out the possibility that the unknown substance was a rare-earth metal.
Shortly after this, Abelson, who had received his Doctor of Science, graduate degree from the university, visited Berkeley for a short vacation and McMillan asked the more able chemist to assist with the separation of the experiment's results. Abelson very quickly observed that whatever was producing the 2.3-day half-life did not have chemistry like any known element and was actually more similar to uranium than a rare-earth metal. This discovery finally allowed the source to be isolated and later, in 1945, led to the classification of the
As research on nuclear fission progressed in early 1939, Edwin McMillan at the Berkeley Radiation Laboratory of the University of California, Berkeley decided to run an experiment bombarding uranium using the powerful 60-inch (1.52 m) cyclotron that had recently been built at the university. The purpose was to separate the various fission products produced by the bombardment by exploiting the enormous force that the fragments gain from their mutual electrical repulsion after fissioning. Although he did not discover anything of note from this, McMillan did observe two new beta decay half-lives in the uranium trioxide target itself, which meant that whatever was producing the radioactivity had not violently repelled each other like normal fission products. He quickly realized that one of the half-lives closely matched the known 23-minute decay period of uranium-239, but the other half-life of 2.3 days was unknown. McMillan took the results of his experiment to chemist and fellow Berkeley professor Emilio Segrè to attempt to isolate the source of the radioactivity. Both scientists began their work using the prevailing theory that element 93 would have similar chemistry to rhenium, but Segrè rapidly determined that McMillan's sample was not at all similar to rhenium. Instead, when he reacted it with hydrogen fluoride (HF) with a strong oxidizing agent present, it behaved much like members of the rare earths. Since these elements comprise a large percentage of fission products, Segrè and McMillan decided that the half-life must have been simply another fission product, titling the paper "An Unsuccessful Search for Transuranium Elements".
However, as more information about fission became available, the possibility that the fragments of nuclear fission could still have been present in the target became more remote. McMillan and several scientists, including Philip H. Abelson, attempted again to determine what was producing the unknown half-life. In early 1940, McMillan realized that his 1939 experiment with Segrè had failed to test the chemical reactions of the radioactive source with sufficient rigor. In a new experiment, McMillan tried subjecting the unknown substance to HF in the presence of a reducing agent, something he had not done before. This reaction resulted in the sample Precipitation (chemistry), precipitating with the HF, an action that definitively ruled out the possibility that the unknown substance was a rare-earth metal.
Shortly after this, Abelson, who had received his Doctor of Science, graduate degree from the university, visited Berkeley for a short vacation and McMillan asked the more able chemist to assist with the separation of the experiment's results. Abelson very quickly observed that whatever was producing the 2.3-day half-life did not have chemistry like any known element and was actually more similar to uranium than a rare-earth metal. This discovery finally allowed the source to be isolated and later, in 1945, led to the classification of the + -> ->[\beta^-][23\ \ce] ->[\beta^-][2.355\ \ce] ''(The times are half-life, half-lives.)''
This proved that the unknown radioactive source originated from the decay of uranium and, coupled with the previous observation that the source was different chemically from all known elements, proved beyond all doubt that a new element had been discovered. McMillan and Abelson published their results in a paper entitled ''Radioactive Element 93'' in the ''Physical Review'' on May 27, 1940. They did not propose a name for the element in the paper, but they soon decided on the name ''neptunium'' since
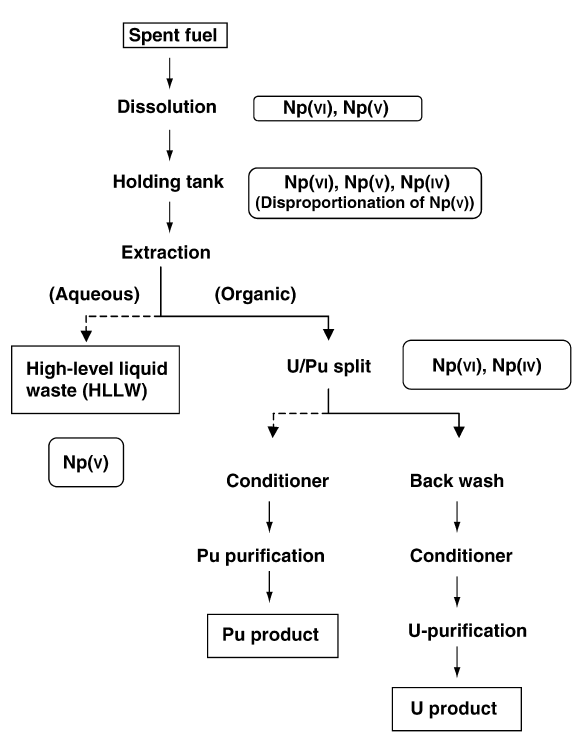
 When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of oxidizing agent, oxidizing or reducing agents, pH of the solution, presence of coordination complex-forming
When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of oxidizing agent, oxidizing or reducing agents, pH of the solution, presence of coordination complex-forming
 A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
^_Np + ^_n -> ^_Np ->[\beta^-][2.117 \ \ce] ^_Pu
238Pu also exists in sizable quantities in
accessed Sept 23, 2006. It is not believed that an actual weapon has ever been constructed using neptunium. As of 2009, the world production of neptunium-237 by commercial power reactors was over 1000 critical masses a year, but to extract the isotope from irradiated fuel elements would be a major industrial undertaking. In September 2002, researchers at the Los Alamos National Laboratory briefly produced the first known nuclear
Neptunium
at ''The Periodic Table of Videos'' (University of Nottingham)
Lab builds world's first neptunium sphere
, U.S. Department of Energy Research News
NLM Hazardous Substances Databank – Neptunium, Radioactive
Neptunium: Human Health Fact Sheet
{{Authority control Neptunium, Chemical elements Actinides Synthetic elements Nuclear materials Pyrophoric materials
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Np and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
93. A radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
metal, neptunium is the first transuranic element. It is named after Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
, the planet beyond Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
in the Solar System, which uranium is named after. A neptunium atom has 93 proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s, ranging from +3 to +7. Like all actinides, it is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
, poisonous, pyrophoric, and capable of accumulating in bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s, which makes the handling of neptunium dangerous.
Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
reactors. While neptunium itself has no commercial uses at present, it is used as a precursor for the formation of plutonium-238, which is in turn used in radioisotope thermal generator
A radioisotope thermoelectric generator (RTG, RITEG), or radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the Decay heat, heat released by the decay of a suitable radioactive material i ...
s to provide electricity for spacecraft
A spacecraft is a vehicle that is designed spaceflight, to fly and operate in outer space. Spacecraft are used for a variety of purposes, including Telecommunications, communications, Earth observation satellite, Earth observation, Weather s ...
. Neptunium has also been used in detectors of high-energy neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s.
The longest-lived isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of neptunium, neptunium-237, is a by-product of nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
production. This isotope, and the isotope neptunium-239, are also found in trace amounts in uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
ores due to neutron capture reactions and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
.
__TOC__
Characteristics
Physical
Neptunium is a hard, silvery,ductile
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
, radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
actinide metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
. In the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, it is located to the right of the actinide uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, to the left of the actinide plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
and below the lanthanide promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust a ...
. Neptunium is a hard metal, having a bulk modulus of 118 GPa, comparable to that of manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
. Neptunium metal is similar to uranium in terms of physical workability. When exposed to air at normal temperatures, it forms a thin oxide layer. This reaction proceeds more rapidly as the temperature increases. Neptunium melts at : this low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
, a property the metal shares with the neighboring element plutonium (which has melting point 639.4 °C), is due to the hybridization of the 5f and 6d orbitals and the formation of directional bonds in the metal. The boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of neptunium is not empirically known and the usually given value of 4174 °C is extrapolated from the vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
of the element. If accurate, this would give neptunium the largest liquid range of any element (3535 K passes between its melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
s).
Neptunium is found in at least three allotropes. Some claims of a fourth allotrope have been made, but they are so far not proven.Yoshida et al., p. 718. This multiplicity of allotropes is common among the actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s. The crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
s of neptunium, protactinium, uranium, and plutonium do not have clear analogs among the lanthanides and are more similar to those of the 3d transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s.
α-neptunium takes on an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
structure, resembling a highly distorted body-centered cubic structure.Lemire, R. J. et al.,''Chemical Thermodynamics of Neptunium and Plutonium'', Elsevier, Amsterdam, 2001. Each neptunium atom is coordinated to four others and the Np–Np bond lengths are 260 pm.Yoshida et al., p. 719. It is the densest of all the actinides and the fifth-densest of all naturally occurring elements, behind only rhenium, platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
, and osmium.Theodore Gray. ''The Elements''. Page 215. α-neptunium has semimetallic properties, such as strong covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
ing and a high electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
, and its metallic physical properties are closer to those of the metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
s than the true metals. Some allotropes of the other actinides also exhibit similar behaviour, though to a lesser degree. The densities of different isotopes of neptunium in the alpha phase are expected to be observably different: α-235Np should have density 20.303 g/cm3; α-236Np, density 20.389 g/cm3; α-237Np, density 20.476 g/cm3.
β-neptunium takes on a distorted tetragonal close-packed structure. Four atoms of neptunium make up a unit cell, and the Np–Np bond lengths are 276 pm. γ-neptunium has a body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal structure#Unit cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
structure and has Np–Np bond length of 297 pm. The γ form becomes less stable with increased pressure, though the melting point of neptunium also increases with pressure. The β-Np/γ-Np/liquid triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
occurs at 725 °C and 3200 MPa.
Alloys
Due to the presence of valence 5f electrons, neptunium and its alloys exhibit a very interesting magnetic behavior, like many other actinides. These can range from the itinerant band-like character characteristic of thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s to the local moment behavior typical of scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, and the lanthanides. This stems from 5f-orbital hybridization with the orbitals of the metal ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s, and the fact that the 5f orbital is relativistically destabilized and extends outwards.Yoshida et al., pp. 719–20. For example, pure neptunium is paramagnetic, Np Al3 is ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ...
, Np Ge3 has no magnetic ordering, and Np Sn3 may be a heavy fermion material. Investigations are underway regarding alloys of neptunium with uranium, americium
Americium is a synthetic element, synthetic chemical element; it has Chemical symbol, symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element e ...
, plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, and iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, so as to recycle long-lived waste isotopes such as neptunium-237 into shorter-lived isotopes more useful as nuclear fuel.
One neptunium-based superconductor alloy has been discovered with formula Np Pd5Al2. This occurrence in neptunium compounds is somewhat surprising because they often exhibit strong magnetism, which usually destroys superconductivity. The alloy has a tetragonal structure with a superconductivity transition temperature of −268.3 °C (4.9 K).
Chemical
Neptunium has five ionicoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
s.
Atomic
A neptunium atom has 93 electrons, arranged in theconfiguration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
noble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
radon; more commonly, only some of the valence electrons will be lost. The electron configuration for the tripositive ion Np3+ is nnbsp;5f4, with the outermost 7s and 6d electrons lost first: this is exactly analogous to neptunium's lanthanide homolog promethium, and conforms to the trend set by the other actinides with their nnbsp;5f''n'' electron configurations in the tripositive state. The first ionization potential
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
of neptunium was measured to be at most in 1974, based on the assumption that the 7s electrons would ionize before 5f and 6d; more recent measurements have refined this to 6.2657 eV.
Isotopes
Twenty-four neptuniumradioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
s have been characterized, with the most stable being 237Np with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 2.14 million years, 236Np with a half-life of 154,000 years, and 235Np with a half-life of 396.1 days. All of the remaining radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has at least four meta state
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
s, with the most stable being 236mNp with a half-life of 22.5 hours. .
The isotopes of neptunium range in atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 219.032 u (219Np) to 244.068 u (244Np), though 221Np has not yet been reported. Most of the isotopes that are lighter than the most stable one, 237Np, decay primarily by electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Th ...
although a sizable number, most notably 229Np and 230Np, also exhibit various levels of decay via alpha emission to become protactinium. 237Np itself, being the beta-stable isobar of mass number 237, decays almost exclusively by alpha emission into 233 Pa, with very rare (occurring only about once in trillions of decays) spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
and cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
(emission of 30Mg to form 207Tl). All of the known isotopes except one that are heavier than this decay exclusively via beta emission
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron t ...
. The lone exception, 240mNp, exhibits a rare (>0.12%) decay by isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
in addition to beta emission. 237Np eventually decays to form bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
-209 and thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
-205, unlike most other common heavy nuclei which decay into isotopes of lead
Lead (82Pb) has four observationally stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay ch ...
. This decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
is known as the neptunium series
In nuclear science a decay chain refers to the predictable series of radioactive decay, radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radionuclide, Radioactive isotopes do not usually decay directly ...
. This decay chain had long been extinct on Earth due to the short half-lives of all of its isotopes above bismuth-209, but is now being resurrected thanks to artificial production of neptunium on the tonne scale.
The isotopes neptunium-235, -236, and -237 are predicted to be fissile
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal Nuclear chain reaction#Fission chain reaction, chain reaction can only be achieved with fissil ...
; only neptunium-237's fissionability has been experimentally shown, with the critical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
being about 60 kg, only about 10 kg more than that of the commonly used uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
. Calculated values of the critical masses of neptunium-235, -236, and -237 respectively are 66.2 kg, 6.79 kg, and 63.6 kg: the neptunium-236 value is even lower than that of plutonium-239
Plutonium-239 ( or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main iso ...
. In particular, 236Np also has a low neutron cross section. Despite this, a neptunium atomic bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear expl ...
has never been built: uranium and plutonium have lower critical masses than 235Np and 237Np, and 236Np is difficult to purify as it is not found in quantity in spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
and is nearly impossible to separate in any significant quantities from 237Np.
Occurrence
The longest-lived isotope of neptunium, 237Np, has a half-life of 2.14 million years, which is more than 2,000 times shorter than theage of the Earth
The age of Earth is estimated to be 4.54 ± 0.05 billion years. This age may represent the age of Earth's accretion (astrophysics), accretion, or Internal structure of Earth, core formation, or of the material from which Earth formed. This dating ...
. Therefore, any primordial neptunium would have decayed in the distant past. After only about 80 million years, the concentration of even the longest-lived isotope, 237Np, would have been reduced to less than one-trillionth (10−12) of its original amount.Yoshida et al., pp. 703–4. Thus neptunium is present in nature only in negligible amounts produced as intermediate decay products of other isotopes.
Trace
Trace may refer to:
Arts and entertainment Music
* ''Trace'' (Son Volt album), 1995
* ''Trace'' (Died Pretty album), 1993
* Trace (band), a Dutch progressive rock band
* ''The Trace'' (album), by Nell
Other uses in arts and entertainment
* ...
amounts of the neptunium isotopes neptunium-237 and -239 are found naturally as decay product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( d ...
s from transmutation reactions in uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
s.Emsley, pp. 345–347. 239Np and 237Np are the most common of these isotopes; they are directly formed from neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
by uranium-238 atoms. These neutrons come from the spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic proc ...
of uranium-238, naturally neutron-induced fission of uranium-235, cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ene ...
of nuclei, and light elements absorbing alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s and emitting a neutron. The half-life of 239Np is very short, although the detection of its much longer-lived daughter
A daughter is a female offspring; a girl or a woman in relation to her parents. Daughterhood is the state, condition or quality of being someone's daughter. The male counterpart is a son. Analogously the name is used in several areas to show r ...
239Pu in nature in 1951 definitively established its natural occurrence. In 1952, 237Np was identified and isolated from concentrates of uranium ore from the Belgian Congo
The Belgian Congo (, ; ) was a Belgian colonial empire, Belgian colony in Central Africa from 1908 until independence in 1960 and became the Republic of the Congo (Léopoldville). The former colony adopted its present name, the Democratic Repu ...
: in these minerals, the ratio of neptunium-237 to uranium is less than or equal to about 10−12 to 1.
Additionally, 240Np must also occur as an intermediate decay product of 244Pu, which has been detected in meteorite dust in marine sediments on Earth.
Most neptunium (and plutonium) now encountered in the environment is due to atmospheric nuclear explosions that took place between the detonation of the first atomic bomb in 1945 and the ratification of the Partial Nuclear Test Ban Treaty
The Partial Test Ban Treaty (PTBT), formally known as the 1963 Treaty Banning Nuclear Weapon Tests in the Atmosphere, in Outer Space and Under Water, prohibited all nuclear weapons testing, test detonations of nuclear weapons except for those co ...
in 1963. The total amount of neptunium released by these explosions and the few atmospheric tests that have been carried out since 1963 is estimated to be around 2500 kg. The overwhelming majority of this is composed of the long-lived isotopes 236Np and 237Np since even the moderately long-lived 235Np (half-life 396 days) would have decayed to less than one-billionth (10−9) its original concentration over the intervening decades. An additional very small amount of neptunium, produced by neutron irradiation of natural uranium in nuclear reactor cooling water, is released when the water is discharged into rivers or lakes. The concentration of 237Np in seawater is approximately 6.5 × 10−5 millibecquerels per liter
The litre ( Commonwealth spelling) or liter ( American spelling) (SI symbols L and l, other symbol used: ℓ) is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cu ...
: this concentration is between 0.1% and 1% that of plutonium.
Once released in the surface environment, in contact with atmospheric oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, neptunium generally oxidizes fairly quickly, usually to the +4 or +5 state. Regardless of its oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
, the element exhibits much greater mobility than the other actinides, largely due to its ability to readily form aqueous solutions with various other elements. In one study comparing the diffusion rates of neptunium(V), plutonium(IV), and americium(III) in sandstone and limestone, neptunium penetrated more than ten times as well as the other elements. Np(V) will also react efficiently in pH levels greater than 5.5 if there are no carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s present and in these conditions it has also been observed to readily bond with quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
. It has also been observed to bond well with goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
, ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when us ...
colloids, and several clays including kaolinite
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina () ...
and smectite
A smectite (; ; ) is a mineral mixture of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secon ...
. Np(V) does not bond as readily to soil particles in mildly acidic conditions as its fellow actinides americium and curium by nearly an order of magnitude. This behavior enables it to migrate rapidly through the soil while in solution without becoming fixed in place, contributing further to its mobility.Atwood, section 4. Np(V) is also readily absorbed by concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
, which because of the element's radioactivity is a consideration that must be addressed when building nuclear waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
storage facilities. When absorbed in concrete, it is reduced to Np(IV) in a relatively short period of time. Np(V) is also reduced by humic acid
Humic substances (HS) are colored relatively recalcitrant organic compounds naturally formed during long-term decomposition and transformation of biomass residues. The color of humic substances varies from bright yellow to light or dark brown lead ...
s if they are present on the surface of goethite, hematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
, and magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
. Np(IV) is less mobile and efficiently adsorbed by tuff
Tuff is a type of rock made of volcanic ash ejected from a vent during a volcanic eruption. Following ejection and deposition, the ash is lithified into a solid rock. Rock that contains greater than 75% ash is considered tuff, while rock co ...
, granodiorite
Granodiorite ( ) is a coarse-grained (phaneritic) intrusive igneous rock similar to granite, but containing more plagioclase feldspar than orthoclase feldspar.
The term banatite is sometimes used informally for various rocks ranging from gra ...
, and bentonite
Bentonite ( ) is an Absorption (chemistry), absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelli ...
; although uptake by the latter is most pronounced in mildly acidic conditions. It also exhibits a strong tendency to bind to colloidal particulates, an effect that is enhanced when in surface soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
with high clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolinite, ). Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impuriti ...
content. The behavior provides an additional aid in the element's observed high mobility.Atwood, section 1.
History
Background and early claims
 When the first
When the first periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
of the elements was published by Dmitri Mendeleev
Dmitri Ivanovich Mendeleev ( ; ) was a Russian chemist known for formulating the periodic law and creating a version of the periodic table of elements. He used the periodic law not only to correct the then-accepted properties of some known ele ...
in the early 1870s, it showed a " — " in place after uranium similar to several other places for then-undiscovered elements. Other subsequent tables of known elements, including a 1913 publication of the known radioactive isotopes by Kasimir Fajans, also show an empty place after uranium, element 92.
Up to and after the discovery of the final component of the atomic nucleus, the neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
in 1932, most scientists did not seriously consider the possibility of elements heavier than uranium. While nuclear theory at the time did not explicitly prohibit their existence, there was little evidence to suggest that they did. However, the discovery of induced radioactivity by Irène and Frédéric Joliot-Curie
Jean Frédéric Joliot-Curie (; ; 19 March 1900 – 14 August 1958) was a French chemist and physicist who received the 1935 Nobel Prize in Chemistry with his wife, Irène Joliot-Curie, for their discovery of induced radioactivity. They were t ...
in late 1933 opened up an entirely new method of researching the elements and inspired a small group of Italian scientists led by Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian and naturalized American physicist, renowned for being the creator of the world's first artificial nuclear reactor, the Chicago Pile-1, and a member of the Manhattan Project ...
to begin a series of experiments involving neutron bombardment. Although the Joliot-Curies' experiment involved bombarding a sample of 27 Al with alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s to produce the radioactive 30 P, Fermi realized that using neutrons, which have no electrical charge, would most likely produce even better results than the positively charged alpha particles. Accordingly, in March 1934 he began systematically subjecting all of the then-known elements to neutron bombardment to determine whether others could also be induced to radioactivity.
After several months of work, Fermi's group had tentatively determined that lighter elements would disperse the energy of the captured neutron by emitting a proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
or alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
and heavier elements would generally accomplish the same by emitting a gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. This latter behavior would later result in the beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
of a neutron into a proton, thus moving the resulting isotope one place up the periodic table. When Fermi's team bombarded uranium, they observed this behavior as well, which strongly suggested that the resulting isotope had an atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
of 93. Fermi was initially reluctant to publicize such a claim, but after his team observed several unknown half-lives in the uranium bombardment products that did not match those of any known isotope, he published a paper entitled ''Possible Production of Elements of Atomic Number Higher than 92'' in June 1934. For element 93, he proposed the name '' ausenium'' (atomic symbol Ao) after the Greek name ''Ausonia'' for Italy.
Several theoretical objections to the claims of Fermi's paper were quickly raised; in particular, the exact process that took place when an atom captured a neutron was not well understood at the time. This and Fermi's accidental discovery three months later that nuclear reactions could be induced by slow neutrons cast further doubt in the minds of many scientists, notably Aristid von Grosse
Aristid von Grosse (January 1905 – July 21, 1985) was a Germans, German nuclear chemist. During his work with Otto Hahn, he got access to waste material from radium production, and with this starting material he was able in 1927 to isolate ...
and Ida Noddack, that the experiment was creating element 93. While von Grosse's claim that Fermi was actually producing protactinium (element 91) was quickly tested and disproved, Noddack's proposal that the uranium had been shattered into two or more much smaller fragments was simply ignored by most because existing nuclear theory did not include a way for this to be possible. Fermi and his team maintained that they were in fact synthesizing a new element, but the issue remained unresolved for several years.
Although the many different and unknown radioactive half-lives in the experiment's results showed that several nuclear reactions were occurring, Fermi's group could not prove that element 93 was being produced unless they could isolate it chemically. They and many other scientists attempted to accomplish this, including Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
and Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
who were among the best radiochemists in the world at the time and supporters of Fermi's claim, but they all failed. Much later, it was determined that the main reason for this failure was because the predictions of element 93's chemical properties were based on a periodic table which lacked the actinide series
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
. This arrangement placed protactinium below tantalum, uranium below tungsten, and further suggested that element 93, at that point referred to as eka-rhenium, should be similar to the group 7 elements, including manganese and rhenium. Thorium, protactinium, and uranium, with their dominant oxidation states of +4, +5, and +6 respectively, fooled scientists into thinking they belonged below hafnium, tantalum, and tungsten, rather than below the lanthanide series, which was at the time viewed as a fluke, and whose members all have dominant +3 states; neptunium, on the other hand, has a much rarer, more unstable +7 state, with +4 and +5 being the most stable. Upon finding that plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
and the other transuranic elements also have dominant +3 and +4 states, along with the discovery of the f-block, the actinide series was firmly established.
While the question of whether Fermi's experiment had produced element 93 was stalemated, two additional claims of the discovery of the element appeared, although unlike Fermi, they both claimed to have observed it in nature. The first of these claims was by Czech engineer Odolen Koblic in 1934 when he extracted a small amount of material from the wash water of heated pitchblende. He proposed the name bohemium for the element, but after being analyzed it turned out that the sample was a mixture of tungsten and vanadium. The other claim, in 1938 by Romanian physicist Horia Hulubei and French chemist Yvette Cauchois, claimed to have discovered the new element via spectroscopy in minerals. They named their element sequanium, but the claim was discounted because the prevailing theory at the time was that if it existed at all, element 93 would not exist naturally. However, as neptunium does in fact occur in nature in trace amounts, as demonstrated when it was found in uranium ore in 1952, it is possible that Hulubei and Cauchois did in fact observe neptunium.
Although by 1938 some scientists, including Niels Bohr, were still reluctant to accept that Fermi had actually produced a new element, he was nevertheless awarded the Nobel Prize in Physics in November 1938 "for his demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons". A month later, the almost totally unexpected discovery of nuclear fission by Hahn, Meitner, and Otto Frisch put an end to the possibility that Fermi had discovered element 93 because most of the unknown half-lives that had been observed by Fermi's team were rapidly identified as those of fission products.
Perhaps the closest of all attempts to produce the missing element 93 was that conducted by the Japanese physicist Yoshio Nishina working with chemist Kenjiro Kimura in 1940, just before the outbreak of the Pacific War in 1941: they bombarded uranium-238, 238U with fast neutrons. However, while slow neutrons tend to induce neutron capture through a (n, γ) reaction, fast neutrons tend to induce a "knock-out" (n, 2n) reaction, where one neutron is added and two more are removed, resulting in the net loss of a neutron. Nishina and Kimura, having tested this technique on 232thorium, Th and successfully produced the known 231Th and its long-lived beta decay daughter 231protactinium, Pa (both occurring in the natural decay chain of uranium-235, 235U), therefore correctly assigned the new 6.75-day half-life activity they observed to the new isotope 237U. They confirmed that this isotope was also a beta emitter and must hence decay to the unknown nuclide 23793. They attempted to isolate this nuclide by carrying it with its supposed lighter congener rhenium, but no beta or alpha decay was observed from the rhenium-containing fraction: Nishina and Kimura thus correctly speculated that the half-life of 23793, like that of 231Pa, was very long and hence its activity would be so weak as to be unmeasurable by their equipment, thus concluding the last and closest unsuccessful search for transuranic elements.
Discovery
 As research on nuclear fission progressed in early 1939, Edwin McMillan at the Berkeley Radiation Laboratory of the University of California, Berkeley decided to run an experiment bombarding uranium using the powerful 60-inch (1.52 m) cyclotron that had recently been built at the university. The purpose was to separate the various fission products produced by the bombardment by exploiting the enormous force that the fragments gain from their mutual electrical repulsion after fissioning. Although he did not discover anything of note from this, McMillan did observe two new beta decay half-lives in the uranium trioxide target itself, which meant that whatever was producing the radioactivity had not violently repelled each other like normal fission products. He quickly realized that one of the half-lives closely matched the known 23-minute decay period of uranium-239, but the other half-life of 2.3 days was unknown. McMillan took the results of his experiment to chemist and fellow Berkeley professor Emilio Segrè to attempt to isolate the source of the radioactivity. Both scientists began their work using the prevailing theory that element 93 would have similar chemistry to rhenium, but Segrè rapidly determined that McMillan's sample was not at all similar to rhenium. Instead, when he reacted it with hydrogen fluoride (HF) with a strong oxidizing agent present, it behaved much like members of the rare earths. Since these elements comprise a large percentage of fission products, Segrè and McMillan decided that the half-life must have been simply another fission product, titling the paper "An Unsuccessful Search for Transuranium Elements".
However, as more information about fission became available, the possibility that the fragments of nuclear fission could still have been present in the target became more remote. McMillan and several scientists, including Philip H. Abelson, attempted again to determine what was producing the unknown half-life. In early 1940, McMillan realized that his 1939 experiment with Segrè had failed to test the chemical reactions of the radioactive source with sufficient rigor. In a new experiment, McMillan tried subjecting the unknown substance to HF in the presence of a reducing agent, something he had not done before. This reaction resulted in the sample Precipitation (chemistry), precipitating with the HF, an action that definitively ruled out the possibility that the unknown substance was a rare-earth metal.
Shortly after this, Abelson, who had received his Doctor of Science, graduate degree from the university, visited Berkeley for a short vacation and McMillan asked the more able chemist to assist with the separation of the experiment's results. Abelson very quickly observed that whatever was producing the 2.3-day half-life did not have chemistry like any known element and was actually more similar to uranium than a rare-earth metal. This discovery finally allowed the source to be isolated and later, in 1945, led to the classification of the
As research on nuclear fission progressed in early 1939, Edwin McMillan at the Berkeley Radiation Laboratory of the University of California, Berkeley decided to run an experiment bombarding uranium using the powerful 60-inch (1.52 m) cyclotron that had recently been built at the university. The purpose was to separate the various fission products produced by the bombardment by exploiting the enormous force that the fragments gain from their mutual electrical repulsion after fissioning. Although he did not discover anything of note from this, McMillan did observe two new beta decay half-lives in the uranium trioxide target itself, which meant that whatever was producing the radioactivity had not violently repelled each other like normal fission products. He quickly realized that one of the half-lives closely matched the known 23-minute decay period of uranium-239, but the other half-life of 2.3 days was unknown. McMillan took the results of his experiment to chemist and fellow Berkeley professor Emilio Segrè to attempt to isolate the source of the radioactivity. Both scientists began their work using the prevailing theory that element 93 would have similar chemistry to rhenium, but Segrè rapidly determined that McMillan's sample was not at all similar to rhenium. Instead, when he reacted it with hydrogen fluoride (HF) with a strong oxidizing agent present, it behaved much like members of the rare earths. Since these elements comprise a large percentage of fission products, Segrè and McMillan decided that the half-life must have been simply another fission product, titling the paper "An Unsuccessful Search for Transuranium Elements".
However, as more information about fission became available, the possibility that the fragments of nuclear fission could still have been present in the target became more remote. McMillan and several scientists, including Philip H. Abelson, attempted again to determine what was producing the unknown half-life. In early 1940, McMillan realized that his 1939 experiment with Segrè had failed to test the chemical reactions of the radioactive source with sufficient rigor. In a new experiment, McMillan tried subjecting the unknown substance to HF in the presence of a reducing agent, something he had not done before. This reaction resulted in the sample Precipitation (chemistry), precipitating with the HF, an action that definitively ruled out the possibility that the unknown substance was a rare-earth metal.
Shortly after this, Abelson, who had received his Doctor of Science, graduate degree from the university, visited Berkeley for a short vacation and McMillan asked the more able chemist to assist with the separation of the experiment's results. Abelson very quickly observed that whatever was producing the 2.3-day half-life did not have chemistry like any known element and was actually more similar to uranium than a rare-earth metal. This discovery finally allowed the source to be isolated and later, in 1945, led to the classification of the actinide series
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
. As a final step, McMillan and Abelson prepared a much larger sample of bombarded uranium that had a prominent 23-minute half-life from 239U and demonstrated conclusively that the unknown 2.3-day half-life increased in strength in concert with a decrease in the 23-minute activity through the following reaction:
:Neptune
Neptune is the eighth and farthest known planet from the Sun. It is the List of Solar System objects by size, fourth-largest planet in the Solar System by diameter, the third-most-massive planet, and the densest giant planet. It is 17 t ...
is the next planet beyond Uranus
Uranus is the seventh planet from the Sun. It is a gaseous cyan-coloured ice giant. Most of the planet is made of water, ammonia, and methane in a Supercritical fluid, supercritical phase of matter, which astronomy calls "ice" or Volatile ( ...
in the Solar System, which uranium is named after. McMillan and Abelson's success compared to Nishina and Kimura's near miss can be attributed to the favorable half-life of 239Np for radiochemical analysis and quick decay of 239U, in contrast to the slower decay of 237U and extremely long half-life of 237Np.
Subsequent developments
It was also realized that the beta decay of 239Np must produce an isotope of element 94 (now calledplutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
), but the quantities involved in McMillan and Abelson's original experiment were too small to isolate and identify plutonium along with neptunium. The discovery of plutonium had to wait until the end of 1940, when Glenn T. Seaborg and his team identified the isotope plutonium-238.
In 1942, Hahn and Fritz Strassmann, and independently Kurt Starke, reported the confirmation of element 93 in Berlin. Hahn's group did not pursue element 94, likely because they were discouraged by McMillan and Abelson's lack of success in isolating it. Since they had access to the stronger cyclotron at Paris at this point, Hahn's group would likely have been able to detect element 94 had they tried, albeit in tiny quantities (a few becquerel (unit), becquerels).
Neptunium's unique radioactive characteristics allowed it to be traced as it moved through various compounds in chemical reactions, at first this was the only method available to prove that its chemistry was different from other elements. As the first isotope of neptunium to be discovered has such a short half-life, McMillan and Abelson were unable to prepare a sample that was large enough to perform chemical analysis of the new element using the technology that was then available. However, after the discovery of the long-lived 237Np isotope in 1942 by Glenn Seaborg and Arthur Wahl, forming weighable amounts of neptunium became a realistic endeavor. Its half-life was initially determined to be about 3 million years (later revised to 2.144 million years), confirming the predictions of Nishina and Kimura of a very long half-life.
Early research into the element was somewhat limited because most of the nuclear physicists and chemists in the United States at the time were focused on the massive effort to research the properties of plutonium as part of the Manhattan Project. Research into the element did continue as a minor part of the project and the first bulk sample of neptunium (as neptunium dioxide) was isolated in 1944.
Much of the research into the properties of neptunium since then has been focused on understanding how to confine it as a portion of nuclear waste. Because it has isotopes with very long half-lives, it is of particular concern in the context of designing confinement facilities that can last for thousands of years. It has found some limited uses as a radioactive tracer and a precursor for various nuclear reactions to produce useful plutonium isotopes. However, most of the neptunium that is produced as a reaction byproduct in nuclear power stations is considered to be a waste product.
Production

Synthesis
The vast majority of the neptunium that currently exists on Earth was produced artificially in nuclear reactions. Neptunium-237 is the most commonly synthesized isotope due to it being the only one that both can be produced vianeutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
and also has a half-life long enough to allow weighable quantities to be easily isolated. It is by far the most common isotope to be utilized in chemical studies of the element.Yoshida et al., p. 700–2.
* When an Uranium-235, 235U atom captures a neutron, it is converted to an excited state of Uranium-236, 236U. About 85.5% of the excited 236U nuclei undergo fission, but the remainder decay to the ground state of 236U by emitting gamma radiation. Further neutron capture forms 237U which has a half-life of 7 days and quickly decays to 237Np through beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
. During beta decay, the excited 237U emits an electron, while the atomic weak interaction converts a neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
to a proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
, thus creating 237Np.
::
* 237U is also produced via an (neutron, n,2n) reaction with Uranium-238, 238U. This only happens with very energetic neutrons.
* 237Np is the product of alpha decay of Americium-241, 241Am, which is produced through neutron irradiation of uranium-238.
Heavier isotopes of neptunium decay quickly, and lighter isotopes of neptunium cannot be produced by neutron capture, so chemical separation of neptunium from cooled spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
gives nearly pure 237Np. The short-lived heavier isotopes 238Np and 239Np, useful as radioactive tracers, are produced through neutron irradiation of 237Np and 238U respectively, while the longer-lived lighter isotopes 235Np and 236Np are produced through irradiation of 235U with proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and deuterons in a cyclotron.
Artificial 237Np metal is usually isolated through a reaction of 237NpF3 with liquid barium or lithium at around 1200 °Celsius, C and is most often extracted from spent nuclear fuel rods in kilogram amounts as a by-product in plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
production.
:2 NpF3 + 3 Ba → 2 Np + 3 BaF2
By weight, neptunium-237 discharges are about 5% as great as plutonium discharges and about 0.05% of spent nuclear fuel discharges. However, even this fraction still amounts to more than fifty tons per year globally.
Purification methods
Recovering uranium and plutonium from spent nuclear fuel for reuse is one of the major processes of the nuclear fuel cycle. As it has a long half-life of just over 2 million years, the alpha decay, alpha emitter 237Np is one of the major isotopes of the minor actinides separated from spent nuclear fuel.Yodshida et al., pp. 704–5. Many separation methods have been used to separate out the neptunium, operating on small and large scales. The small-scale purification operations have the goals of preparing pure neptunium as a precursor (chemistry), precursor of metallic neptunium and its compounds, and also to isolate and preconcentrate neptunium in samples for analysis. Most methods that separate neptunium ions exploit the differing chemical behaviour of the differing oxidation states of neptunium (from +3 to +6 or sometimes even +7) in solution. Among the methods that are or have been used are: solvent extraction (chemistry), extraction (using various extractants, usually denticity, multidentate β-diketone derivatives, organophosphorus compounds, and amine compounds), chromatography using various ion exchange, ion-exchange or chelation, chelating resins, coprecipitation (possible matrix (chemical analysis), matrices include lanthanum(III) fluoride, LaF3, bismuth phosphate, BiPO4, barium sulfate, BaSO4, iron(III) hydroxide, Fe(OH)3, and manganese(IV) oxide, MnO2), electroplating, electrodeposition, and biotechnology, biotechnological methods.Yoshida et al., pp. 705–17. Currently, commercial reprocessing plants use the Purex process, involving the solvent extraction of uranium and plutonium with tributyl phosphate.Yoshida et al., p. 710.Chemistry and compounds
Solution chemistry
 When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of oxidizing agent, oxidizing or reducing agents, pH of the solution, presence of coordination complex-forming
When it is in an aqueous solution, neptunium can exist in any of its five possible oxidation states (+3 to +7) and each of these show a characteristic color. The stability of each oxidation state is strongly dependent on various factors, such as the presence of oxidizing agent, oxidizing or reducing agents, pH of the solution, presence of coordination complex-forming ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s, and even the concentration of neptunium in the solution.Yoshida et al., pp. 752–4.
In acid (chemistry), acidic solutions, the neptunium(III) to neptunium(VII) ions exist as Np3+, Np4+, , , and . In base (chemistry), basic solutions, they exist as the oxides and hydroxides Np(OH)3, NpO2, NpO2OH, NpO2(OH)2, and . Not as much work has been done to characterize neptunium in basic solutions. Np3+ and Np4+ can easily be reduced and oxidized to each other, as can and .Yoshida et al., p. 759.
;Neptunium(III)
Np(III) or Np3+ exists as hydrated complexes in acidic solutions, . It is a dark blue-purple and is analogous to its lighter congener (chemistry), congener, the pink rare-earth metal, rare-earth ion promethium, Pm3+. In the presence of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, it is quickly oxidized to Np(IV) unless strong reducing agents are also present. Nevertheless, it is the second-least easily hydrolysis, hydrolyzed neptunium ion in water, forming the NpOH2+ ion. Np3+ is the predominant neptunium ion in solutions of pH 4–5.Yoshida et al., p. 766–70.
;Neptunium(IV)
Np(IV) or Np4+ is pale yellow-green in acidic solutions, where it exists as hydrated complexes (). It is quite unstable to hydrolysis in acidic aqueous solutions at pH 1 and above, forming NpOH3+. In basic solutions, Np4+ tends to hydrolyze to form the neutral neptunium(IV) hydroxide (Np(OH)4) and neptunium(IV) oxide (NpO2).
;Neptunium(V)
Np(V) or is green-blue in aqueous solution, in which it behaves as a strong Lewis acid. It is a stable ion and is the most common form of neptunium in aqueous solutions. Unlike its neighboring homologues and , does not spontaneously disproportionation, disproportionate except at very low pH and high concentration:
:2 + 4 H+ ⇌ Np4+ + + 2 H2O
It hydrolyzes in basic solutions to form NpO2OH and .
;Neptunium(VI)
Np(VI) or , the neptunyl ion, shows a light pink or reddish color in an acidic solution and yellow-green otherwise. It is a strong Lewis acid and is the main neptunium ion encountered in solutions of pH 3–4. Though stable in acidic solutions, it is quite easily reduced to the Np(V) ion, and it is not as stable as the homologous hexavalent ions of its neighbours uranium and plutonium (the uranyl and plutonyl ions). It hydrolyzes in basic solutions to form the oxo and hydroxo ions NpO2OH+, , and .
;Neptunium(VII)
Np(VII) is dark green in a strongly Base (chemistry), basic solution. Though its chemical formula in basic solution is frequently cited as , this is a simplification and the real structure is probably closer to a hydroxo species like . Np(VII) was first prepared in basic solution in 1967. In strongly acid (chemistry), acidic solution, Np(VII) is found as ; water quickly reduces this to Np(VI). Its hydrolysis products are uncharacterized.
Hydroxides
The oxides and hydroxides of neptunium are closely related to its ions. In general, Np hydroxides at various oxidation levels are less stable than the actinides before it on the periodic table such as thorium and uranium and more stable than those after it such as plutonium and americium. This phenomenon is because the stability of an ion increases as the ratio of atomic number to the radius of the ion increases. Thus actinides higher on the periodic table will more readily undergo hydrolysis. Neptunium(III) hydroxide is quite stable in acidic solutions and in environments that lack oxygen, but it will rapidly oxidize to the IV state in the presence of air. It is not soluble in water. Np(IV) hydroxides exist mainly as the electrically neutral Np(OH)4 and its mild solubility in water is not affected at all by the pH of the solution. This suggests that the other Np(IV) hydroxide, , does not have a significant presence. Because the Np(V) ion is very stable, it can only form a hydroxide in high acidity levels. When placed in a 0.1 molar concentration, M sodium perchlorate solution, it does not react significantly for a period of months, although a higher molar concentration of 3.0 M will result in it reacting to the solid hydroxide NpO2OH almost immediately. Np(VI) hydroxide is more reactive but it is still fairly stable in acidic solutions. It will form the compound NpO3· H2O in the presence of ozone under various carbon dioxide pressures. Np(VII) has not been well-studied and no neutral hydroxides have been reported. It probably exists mostly as .Oxides
Three anhydrous neptunium oxides have been reported, neptunium(IV) oxide, NpO2, Np2O5, and Np3O8, though some studies have stated that only the first two of these exist, suggesting that claims of Np3O8 are actually the result of mistaken analysis of Np2O5. However, as the full extent of the reactions that occur between neptunium and oxygen has yet to be researched, it is not certain which of these claims is accurate. Although neptunium oxides have not been produced with neptunium in oxidation states as high as those possible with the adjacent actinide uranium, neptunium oxides are more stable at lower oxidation states. This behavior is illustrated by the fact that NpO2 can be produced by simply burning neptunium salts of oxyacids in air.Yoshida et al., 724–726. The greenish-brown NpO2 is very stable over a large range of pressures and temperatures and does not undergo phase transitions at low temperatures. It does show a phase transition from face-centered cubic to orthorhombic at around 33–37 GPa, although it returns to its original phase when pressure is released. It remains stable under oxygen pressures up to 2.84 MPa and temperatures up to 400 °C. Np2O5 is black-brown in color and monoclinic with a lattice size of 418×658×409 picometres. It is relatively unstable and decomposes to NpO2 and O2 at 420–695 °C. Although Np2O5 was initially subject to several studies that claimed to produce it with mutually contradictory methods, it was eventually prepared successfully by heating neptunium peroxide to 300–350 °C for 2–3 hours or by heating it under a layer of water in an ampoule at 180 °C. Neptunium also forms a large number of oxide compounds with a wide variety of elements, although the neptunate oxides formed with alkali metals and alkaline earth metals have been by far the most studied. Ternary neptunium oxides are generally formed by reacting NpO2 with the oxide of another element or by precipitating from an alkaline solution. Lithium, Li5NpO6 has been prepared by reacting Li2O and NpO2 at 400 °C for 16 hours or by reacting Li2O2 with NpO3 · H2O at 400 °C for 16 hours in a quartz tube and flowing oxygen. Alkali neptunate compounds Potassium, K3NpO5, Caesium, Cs3NpO5, and Rubidium, Rb3NpO5 are all produced by a similar reaction: :NpO2 + 3 MO2 → M3NpO5 (M = K, Cs, Rb) The oxide compounds KNpO4, CsNpO4, and RbNpO4 are formed by reacting Np(VII) () with a compound of the alkali metal nitrate and ozone. Additional compounds have been produced by reacting NpO3 and water with solid alkali and alkaline peroxides at temperatures of 400–600 °C for 15–30 hours. Some of these include Ba3(NpO5)2, Ba2Sodium, NaNpO6, and Ba2LiNpO6. Also, a considerable number of hexavalent neptunium oxides are formed by reacting solid-state NpO2 with various alkali or alkaline earth oxides in an environment of flowing oxygen. Many of the resulting compounds also have an equivalent compound that substitutes uranium for neptunium. Some compounds that have been characterized include Na2Np2O7, Na4NpO5, Na6NpO6, and Na2NpO4. These can be obtained by heating different combinations of NpO2 and Na2O to various temperature thresholds and further heating will also cause these compounds to exhibit different neptunium allotropes. The lithium neptunate oxides Li6NpO6 and Li4NpO5 can be obtained with similar reactions of NpO2 and Li2O.Yoshida et al, pp. 728–730. A large number of additional alkali and alkaline neptunium oxide compounds such as Cs4Np5O17 and Cs2Np3O10 have been characterized with various production methods. Neptunium has also been observed to form ternary oxides with many additional elements in Group (periodic table), groups 3 through 7, although these compounds are much less well studied.Halides
Although neptunium halide compounds have not been nearly as well studied as its oxides, a fairly large number have been successfully characterized. Of these, neptunium fluorides have been the most extensively researched, largely because of their potential use in separating the element from nuclear waste products. Four binary neptunium fluoride compounds, Npfluorine, F3, NpF4, NpF5, and NpF6, have been reported. The first two are fairly stable and were first prepared in 1947 through the following reactions: :NpO2 + H2 + 3 HF → NpF3 + 2 H2O (400°C) :NpF3 + O2 + HF → NpF4 + H2O (400°C) Later, NpF4 was obtained directly by heating NpO2 to various temperatures in mixtures of either hydrogen fluoride or pure fluorine gas. NpF5 is much more difficult to form and most known preparation methods involve reacting NpF4 or NpF6 compounds with various other fluoride compounds. NpF5 will decompose into NpF4 and NpF6 when heated to around 320 °C.Yoshida et al, pp. 730–736. NpF6 or neptunium hexafluoride is extremely volatile, as are its adjacent actinide compounds uranium hexafluoride (UF6) and plutonium hexafluoride (PuF6). This volatility has attracted a large amount of interest to the compound in an attempt to devise a simple method for extracting neptunium from spent nuclear power station fuel rods. NpF6 was first prepared in 1943 by reacting NpF3 and gaseous fluorine at very high temperatures and the first bulk quantities were obtained in 1958 by heating NpF4 and dripping pure fluorine on it in a specially prepared apparatus. Additional methods that have successfully produced neptunium hexafluoride include reacting bromine trifluoride, BrF3 and bromine pentafluoride, BrF5 with NpF4 and by reacting several different neptunium oxide and fluoride compounds with anhydrous hydrogen fluorides. Four neptunium Oxohalide, oxyfluoride compounds, NpO2F, NpOF3, NpO2F2, and NpOF4, have been reported, although none of them have been extensively studied. NpO2F2 is a pinkish solid and can be prepared by reacting NpO3 · H2O and Np2F5 with pure fluorine at around 330 °C. NpOF3 and NpOF4 can be produced by reacting neptunium oxides with anhydrous hydrogen fluoride at various temperatures. Neptunium also forms a wide variety of fluoride compounds with various elements. Some of these that have been characterized include CsNpF6, Rb2NpF7, Na3NpF8, and K3NpO2F5. Two neptunium chlorides, Npchlorine, Cl3 and NpCl4, have been characterized. Although several attempts to obtain NpCl5 have been made, they have not been successful. NpCl3 is produced by reducing neptunium dioxide with hydrogen and carbon tetrachloride (carbon, CCl4) and NpCl4 by reacting a neptunium oxide with CCl4 at around 500 °C. Other neptunium chloride compounds have also been reported, including NpOCl2, Cs2NpCl6, Cs3NpO2Cl4, and Cs2NaNpCl6. Neptunium bromides Npbromine, Br3 and NpBr4 have also been produced; the latter by reacting aluminium bromide with NpO2 at 350 °C and the former in an almost identical procedure but with zinc present. The neptunium iodide Npiodine, I3 has also been prepared by the same method as NpBr3.Yoshida et al, pp. 736–738.Chalcogenides, pnictides, and carbides
Neptunium chalcogen and pnictogen compounds have been well studied primarily as part of research into their electronic and magnetic properties and their interactions in the natural environment. Pnictide and carbide compounds have also attracted interest because of their presence in the fuel of several advanced nuclear reactor designs, although the latter group has not had nearly as much research as the former.Yoshida et al, pp. 739–742. ;Chalcogenides A wide variety of neptunium sulfide compounds have been characterized, including the pure sulfide compounds Npsulfur, S, NpS3, Np2S5, Np3S5, Np2S3, and Np3S4. Of these, Np2S3, prepared by reacting NpO2 with hydrogen sulfide and carbon disulfide at around 1000 °C, is the most well-studied and three allotropic forms are known. The α form exists up to around 1230 °C, the β up to 1530 °C, and the γ form, which can also exist as Np3S4, at higher temperatures. NpS can be produced by reacting Np2S3 and neptunium metal at 1600 °C and Np3S5 can be prepared by the decomposition of Np2S3 at 500 °C or by reacting sulfur and neptunium hydride at 650 °C. Np2S5 is made by heating a mixture of Np3S5 and pure sulfur to 500 °C. All of the neptunium sulfides except for the β and γ forms of Np2S3 are isostructural with the equivalent uranium sulfide and several, including NpS, α−Np2S3, and β−Np2S3 are also isostructural with the equivalent plutonium sulfide. The oxysulfides NpOS, Np4O4S, and Np2O2S have also been produced, although the latter three have not been well studied. NpOS was first prepared in 1985 by vacuum sealing NpO2, Np3S5, and pure sulfur in a quartz tube and heating it to 900 °C for one week. Neptunium selenide compounds that have been reported include Npselenium, Se, NpSe3, Np2Se3, Np2Se5, Np3Se4, and Np3Se5. All of these have only been obtained by heating neptunium hydride and selenium metal to various temperatures in a vacuum for an extended period of time and Np2Se3 is only known to exist in the γ allotrope at relatively high temperatures. Two neptunium oxyselenide compounds are known, NpOSe and Np2O2Se, are formed with similar methods by replacing the neptunium hydride with neptunium dioxide. The known neptunium telluride (chemistry), telluride compounds Nptellurium, Te, NpTe3, Np3Te4, Np2Te3, and Np2O2Te are formed by similar procedures to the selenides and Np2O2Te is isostructural to the equivalent uranium and plutonium compounds. No neptunium−polonium compounds have been reported. ;Pnictides and carbides Neptunium nitride (Npnitrogen, N) was first prepared in 1953 by reacting neptunium hydride and ammonia gas at around 750 °C in a quartz capillary tube. Later, it was produced by reacting different mixtures of nitrogen and hydrogen with neptunium metal at various temperatures. It has also been produced by the reduction of neptunium dioxide with Diatomic molecule, diatomic nitrogen gas at 1550 °C. NpN is Isomorphism (crystallography), isomorphous with Uranium nitride, uranium mononitride (UN) and Plutonium nitride, plutonium mononitride (PuN) and has a melting point of 2830 °C under a nitrogen pressure of around 1 MPa. Two neptunium phosphide compounds have been reported, Npphosphorus, P and Np3P4. The first has a face centered cubic structure and is prepared by converting neptunium metal to a powder and then reacting it with phosphine gas at 350 °C. Np3P4 can be produced by reacting neptunium metal with red phosphorus at 740 °C in a vacuum and then allowing any extra phosphorus to sublimation (phase transition), sublimate away. The compound is non-reactive with water but will react with nitric acid to produce Np(IV) solution.Yoshida et al, pp. 742–744. Three neptunium arsenide compounds have been prepared, Nparsenic, As, NpAs2, and Np3As4. The first two were first produced by heating arsenic and neptunium hydride in a vacuum-sealed tube for about a week. Later, NpAs was also made by confining neptunium metal and arsenic in a vacuum tube, separating them with a quartz membrane, and heating them to just below neptunium's melting point of 639 °C, which is slightly higher than the arsenic's sublimation point of 615 °C. Np3As4 is prepared by a similar procedure using iodine as a Chemical transport reaction, transporting agent. NpAs2 crystals are brownish gold and Np3As4 is black. The neptunium antimonide compound Npantimony, Sb was produced in 1971 by placing equal quantities of both elements in a vacuum tube, heating them to the melting point of antimony, and then heating it further to 1000 °C for sixteen days. This procedure also produced trace amounts of an additional antimonide compound Np3Sb4. One neptunium-bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
compound, NpBi, has also been reported.
The neptunium carbides Npcarbon, C, Np2C3, and NpC2 (tentative) have been reported, but have not characterized in detail despite the high importance and utility of actinide carbides as advanced nuclear reactor fuel. NpC is a non-stoichiometric compound, and could be better labelled as NpC''x'' (0.82 ≤ ''x'' ≤ 0.96). It may be obtained from the reaction of neptunium hydride with graphite at 1400 °C or by heating the constituent elements together in an electric arc furnace using a tungsten electrode. It reacts with excess carbon to form pure Np2C3. NpC2 is formed from heating NpO2 in a graphite crucible at 2660–2800 °C.
Other inorganic
;Hydrides Neptunium reacts with hydrogen in a similar manner to its neighbor plutonium, forming the hydrides NpH2+''x'' (face-centered cubic) and NpH3 (hexagonal crystal system, hexagonal). These are isostructural with the corresponding plutonium hydrides, although unlike PuH2+''x'', the lattice parameters of NpH2+''x'' become greater as the hydrogen content (''x'') increases. The hydrides require extreme care in handling as they decompose in a vacuum at 300 °C to form finely divided neptunium metal, which is pyrophoric.Yoshida et al., pp. 722–4. ;Phosphates, sulfates, and carbonates Being chemically stable, neptunium phosphates have been investigated for potential use in immobilizing nuclear waste. Neptunium pyrophosphate (α-NpP2O7), a green solid, has been produced in the reaction between neptunium dioxide and boron phosphate at 1100 °C, though neptunium(IV) phosphate has so far remained elusive. The series of compounds NpM2(PO4)3, where M is an alkali metal (lithium, Li, sodium, Na, potassium, K, rubidium, Rb, or caesium, Cs), are all known. Some neptunium sulfates have been characterized, both aqueous and solid and at various oxidation states of neptunium (IV through VI have been observed). Additionally, neptuniumcarbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s have been investigated to achieve a better understanding of the behavior of neptunium in geological repository, geological repositories and the environment, where it may come into contact with carbonate and bicarbonate aqueous solutions and form soluble complexes.Yoshida et al., pp. 744–5.
Organometallic
 A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to
A few organoneptunium compounds are known and chemically characterized, although not as many as for organouranium compound, uranium due to neptunium's scarcity and radioactivity. The most well known organoneptunium compounds are the cyclopentadienyl and cyclooctatetraenyl compounds and their derivatives.Yoshida et al., pp. 750–2. The trivalent cyclopentadienyl compound Np(C5H5)3·tetrahydrofuran, THF was obtained in 1972 from reacting Np(C5H5)3Cl with sodium, although the simpler Np(C5H5) could not be obtained. Tetravalent neptunium cyclopentadienyl, a reddish-brown complex, was synthesized in 1968 by reacting neptunium(IV) chloride with potassium cyclopentadienide:
:NpCl4 + 4 KC5H5 → Np(C5H5)4 + 4 KCl
It is soluble in benzene and Tetrahydrofuran, THF, and is less sensitive to oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and water than plutonium, Pu(C5H5)3 and americium, Am(C5H5)3. Other Np(IV) cyclopentadienyl compounds are known for many ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s: they have the general formula (C5H5)3NpL, where L represents a ligand.
Neptunocene, Np(C8H8)2, was synthesized in 1970 by reacting neptunium(IV) chloride with K2(C8H8). It is Isomorphism (crystallography), isomorphous to uranocene and plutonocene, and they behave chemically identically: all three compounds are insensitive to water or dilute bases but are sensitive to air, reacting quickly to form oxides, and are only slightly soluble in benzene and toluene. Other known neptunium cyclooctatetraenyl derivatives include Np(RC8H7)2 (R = ethanol, butanol) and KNp(C8H8)·2THF, which is isostructural to the corresponding plutonium compound. In addition, neptunium hydrocarbyls have been prepared, and solvated triiodide complexes of neptunium are a precursor to many organoneptunium and inorganic neptunium compounds.
Coordination complexes
There is much interest in the coordination chemistry of neptunium, because its five oxidation states all exhibit their own distinctive chemical behavior, and the coordination chemistry of the actinides is heavily influenced by the actinide contraction (the greater-than-expected decrease in ionic radii across the actinide series, analogous to the lanthanide contraction).Yoshida et al., pp. 745–750.Solid state
Few neptunium(III) coordination compounds are known, because Np(III) is readily oxidized by atmospheric oxygen while in aqueous solution. However, sodium formaldehyde sulfoxylate can reduce Np(IV) to Np(III), stabilizing the lower oxidation state and forming various sparingly soluble Np(III) coordination complexes, such as ·11H2O, ·H2O, and . Many neptunium(IV) coordination compounds have been reported, the first one being , which is isostructural with the analogous uranium(IV) coordination compound. Other Np(IV) coordination compounds are known, some involving other metals such as cobalt (·8H2O, formed at 400 K) and copper (·6H2O, formed at 600 K). Complex nitrate compounds are also known: the experimenters who produced them in 1986 and 1987 obtained single crystals by slow evaporation of the Np(IV) solution at ambient temperature in concentrated nitric acid and excess 2,2′-pyrimidine. The coordination chemistry of neptunium(V) has been extensively researched due to the presence of cation–cation interactions in the solid state, which had been already known for actinyl ions. Some known such compounds include the neptunyl dimer (chemistry), dimer ·8H2O and neptunium glycolate, both of which form green crystals. Neptunium(VI) compounds range from the simple oxalate (which is unstable, usually becoming Np(IV)) to such complicated compounds as the green . Extensive study has been performed on compounds of the form , where M represents a monovalent cation and An is either uranium, neptunium, or plutonium. Since 1967, when neptunium(VII) was discovered, some coordination compounds with neptunium in the +7 oxidation state have been prepared and studied. The first reported such compound was initially characterized as ·''n''H2O in 1968, but was suggested in 1973 to actually have the formula ·2H2O based on the fact that Np(VII) occurs as in aqueous solution. This compound forms dark green prismatic crystals with maximum edge length 0.15–0.4 millimeter, mm.In aqueous solution
Most neptunium coordination complexes known in solution involve the element in the +4, +5, and +6 oxidation states: only a few studies have been done on neptunium(III) and (VII) coordination complexes.Yoshida et al., pp. 771–82. For the former, NpX2+ and (X = chlorine, Cl, bromine, Br) were obtained in 1966 in concentrated lithium chloride, LiCl and lithium bromide, LiBr solutions, respectively: for the latter, 1970 experiments discovered that the ion could form sulfate complexes in acidic solutions, such as and ; these were found to have higher equilibrium constant, stability constants than the neptunyl ion (). A great many complexes for the other neptunium oxidation states are known: the inorganic ligands involved are the halides, iodate, azide, nitride, nitrate, thiocyanate, sulfate,carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
, Chromate ion, chromate, and phosphate. Many organic ligands are known to be able to be used in neptunium coordination complexes: they include acetate, propionate, glycolate, lactic acid, lactate, oxalate, malonate, phthalate, mellitate, and citrate.
Analogously to its neighbours, uranium and plutonium, the order of the neptunium ions in terms of complex formation ability is Np4+ > ≥ Np3+ > . (The relative order of the middle two neptunium ions depends on the ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s and solvents used.) The stability sequence for Np(IV), Np(V), and Np(VI) complexes with monovalent inorganic ligands is fluoride, F− > dihydrogen phosphate, > thiocyanate, SCN− > nitrate, > chloride, Cl− > perchlorate, ; the order for divalent inorganic ligands is carbonate, > Monohydrogen phosphate, > sulfate, . These follow the strengths of the corresponding acids. The divalent ligands are more strongly complexing than the monovalent ones. can also form the complex ions [] (M = Al, gallium, Ga, scandium, Sc, indium, In, iron, Fe, chromium, Cr, rhodium, Rh) in perchloric acid solution: the strength of interaction between the two cations follows the order Fe > In > Sc > Ga > Al. The neptunyl and uranyl ions can also form a complex together.
Applications
Precursor in plutonium-238 production
An important use of 237Np is as a precursor in plutonium-238 production, where it is irradiated with neutrons to form Plutonium-238, 238Pu, an alpha emitter forradioisotope thermal generator
A radioisotope thermoelectric generator (RTG, RITEG), or radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the Decay heat, heat released by the decay of a suitable radioactive material i ...
s for spacecraft and military applications. 237Np will capture a neutron to form 238Np and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
with a half-life of just over two days to 238Pu.
:spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
but would have to be separated from other isotopes of plutonium.Yoshida et al., pp. 702–3. Irradiating neptunium-237 with electron beams, provoking bremsstrahlung, also produces quite pure samples of the isotope plutonium-236, useful as a tracer to determine plutonium concentration in the environment.
Nuclear weapons
Neptunium is fissionable, and could theoretically be used as fuel in a fast-neutron reactor or a nuclear weapon, with acritical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
of around 60 kilograms. In 1992, the U.S. Department of Energy declassified the statement that neptunium-237 "can be used for a nuclear explosive device"."Restricted Data Declassification Decisions from 1946 until Present"accessed Sept 23, 2006. It is not believed that an actual weapon has ever been constructed using neptunium. As of 2009, the world production of neptunium-237 by commercial power reactors was over 1000 critical masses a year, but to extract the isotope from irradiated fuel elements would be a major industrial undertaking. In September 2002, researchers at the Los Alamos National Laboratory briefly produced the first known nuclear
critical mass
In nuclear engineering, critical mass is the minimum mass of the fissile material needed for a sustained nuclear chain reaction in a particular setup. The critical mass of a fissionable material depends upon its nuclear properties (specific ...
using a significant fraction of neptunium, in combination with shells of enriched uranium (uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
), discovering that the critical mass of a bare sphere of neptunium-237 "ranges from kilogram weights in the high fifties to low sixties," showing that it "is about as good a bomb material as [uranium-235]." The United States Federal government made plans in March 2004 to move America's supply of separated neptunium to a nuclear-waste disposal site in Nevada.
Physics
237Np is used in devices for detecting high-energy (MeV) neutrons.Role in nuclear waste
Neptunium accumulates in commercial household ionization-chamber smoke detectors from decay of the (typically) 0.2 microgram of americium-241 initially present as a source of ionizing radiation. With a half-life of 432 years, the americium-241 in an smoke detector#Design, ionization smoke detector includes about 3% neptunium after 20 years, and about 15% after 100 years. Under Redox, oxidizing conditions, neptunium-237 is the most mobileactinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
in the deep geological repository environment of the Yucca Mountain project in Nevada. This makes it and its predecessors such as americium-241 candidates of interest for destruction by nuclear transmutation. Due to its long half-life, neptunium will become the major contributor of the total radiotoxicity at Yucca Mountain in 10,000 years. As it is unclear what happens to the non-reprocessed spent fuel containment in that long time span, an extraction and transmutation of neptunium after spent fuel reprocessing could help to minimize the contamination of the environment if the nuclear waste could be mobilized after several thousand years.
Biological role and precautions
Neptunium does not have a biological role, as it has a short half-life and occurs only in small traces naturally. Animal tests show it to be absorbed poorly (~1%) via the digestive tract. When injected it concentrates in the bones, from which it is slowly released. Finely divided neptunium metal presents a fire hazard because neptunium is pyrophoric; small grains will ignite spontaneously in air at room temperature.References
Bibliography
* * * * * *Literature
* ''Guide to the Elements – Revised Edition'', Albert Stwertka, (Oxford University Press; 1998) * Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer-Verlag, Dordrecht 2006, . * * Eric Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011, .External links
Neptunium
at ''The Periodic Table of Videos'' (University of Nottingham)
Lab builds world's first neptunium sphere
, U.S. Department of Energy Research News
NLM Hazardous Substances Databank – Neptunium, Radioactive
Neptunium: Human Health Fact Sheet
{{Authority control Neptunium, Chemical elements Actinides Synthetic elements Nuclear materials Pyrophoric materials