mercuric oxide on:
[Wikipedia]
[Google]
[Amazon]
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the
 The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by
The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by
 Mercury oxide is a highly toxic substance which can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion. The substance is irritating to the eyes, the skin and the respiratory tract and may have effects on the kidneys, resulting in kidney impairment. In the food chain important to humans,
Mercury oxide is a highly toxic substance which can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion. The substance is irritating to the eyes, the skin and the respiratory tract and may have effects on the kidneys, resulting in kidney impairment. In the food chain important to humans,
National Pollutant Inventory – Mercury and compounds fact sheet
{{Authority control Oxides Mercury(II) compounds Inorganic compounds
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found.
History
An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' It was historically called red precipitate (as opposed to white precepitate being the mercuric amidochloride). In 1774,Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, Unitarian, Natural philosophy, natural philosopher, English Separatist, separatist theologian, Linguist, grammarian, multi-subject educator and Classical libera ...
discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(rather, Priestley called it " dephlogisticated air," as that was the paradigm
In science and philosophy, a paradigm ( ) is a distinct set of concepts or thought patterns, including theories, research methods, postulates, and standards for what constitute legitimate contributions to a field. The word ''paradigm'' is Ancient ...
that he was working under at the time).
Synthesis and reactions

 The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by
The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
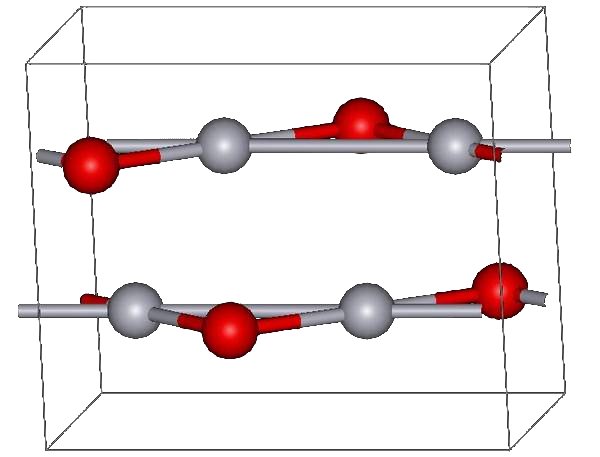
of Hg(NO3)2. The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali. The difference in color is due to particle size; both forms have the same structure consisting of near linear O-Hg-O units linked in zigzag chains with an Hg-O-Hg angle of 108°.
HgO is soluble in many conventional strong acids through protonation of the anion. The exceptions include acids which form insoluble mercury(II) salts, like mercury(II) iodide in the case of hydroiodic acid. Dissolution is also possible through complexation of the cation; e.g. cyanide ligands form stable water soluble mercury(II) complexes.
Structure
Under atmospheric pressure mercuric oxide has two crystalline forms: one is called montroydite ( orthorhombic, 2/m 2/m 2/m, Pnma), and the second is analogous to the sulfide mineralcinnabar
Cinnabar (; ), or cinnabarite (), also known as ''mercurblende'' is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and is t ...
(hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
,
hP6, P3221); both are characterized by Hg-O chains. At pressures above 10 GPa both structures convert to a tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the Cube (geometry), cube becomes a rectangular Pri ...
form.
Uses
Mercury oxide is sometimes used in the production of mercury as it decomposes quite easily. When it decomposes, oxygen gas is generated. It is also used as a material forcathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
s in mercury batteries.
Health issues
 Mercury oxide is a highly toxic substance which can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion. The substance is irritating to the eyes, the skin and the respiratory tract and may have effects on the kidneys, resulting in kidney impairment. In the food chain important to humans,
Mercury oxide is a highly toxic substance which can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion. The substance is irritating to the eyes, the skin and the respiratory tract and may have effects on the kidneys, resulting in kidney impairment. In the food chain important to humans, bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. T ...
takes place, specifically in aquatic organisms. The substance is banned as a pesticide in the EU.
Evaporation at 20 °C is negligible. HgO decomposes on exposure to light or on heating above 500 °C. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. Mercury(II) oxide reacts violently with reducing agents, chlorine, hydrogen peroxide, magnesium (when heated), disulfur dichloride and hydrogen trisulfide. Shock-sensitive compounds are formed with metals and elements such as sulfur and phosphorus.
References
External links
National Pollutant Inventory – Mercury and compounds fact sheet
{{Authority control Oxides Mercury(II) compounds Inorganic compounds