leaving group on:
[Wikipedia]
[Google]
[Amazon]
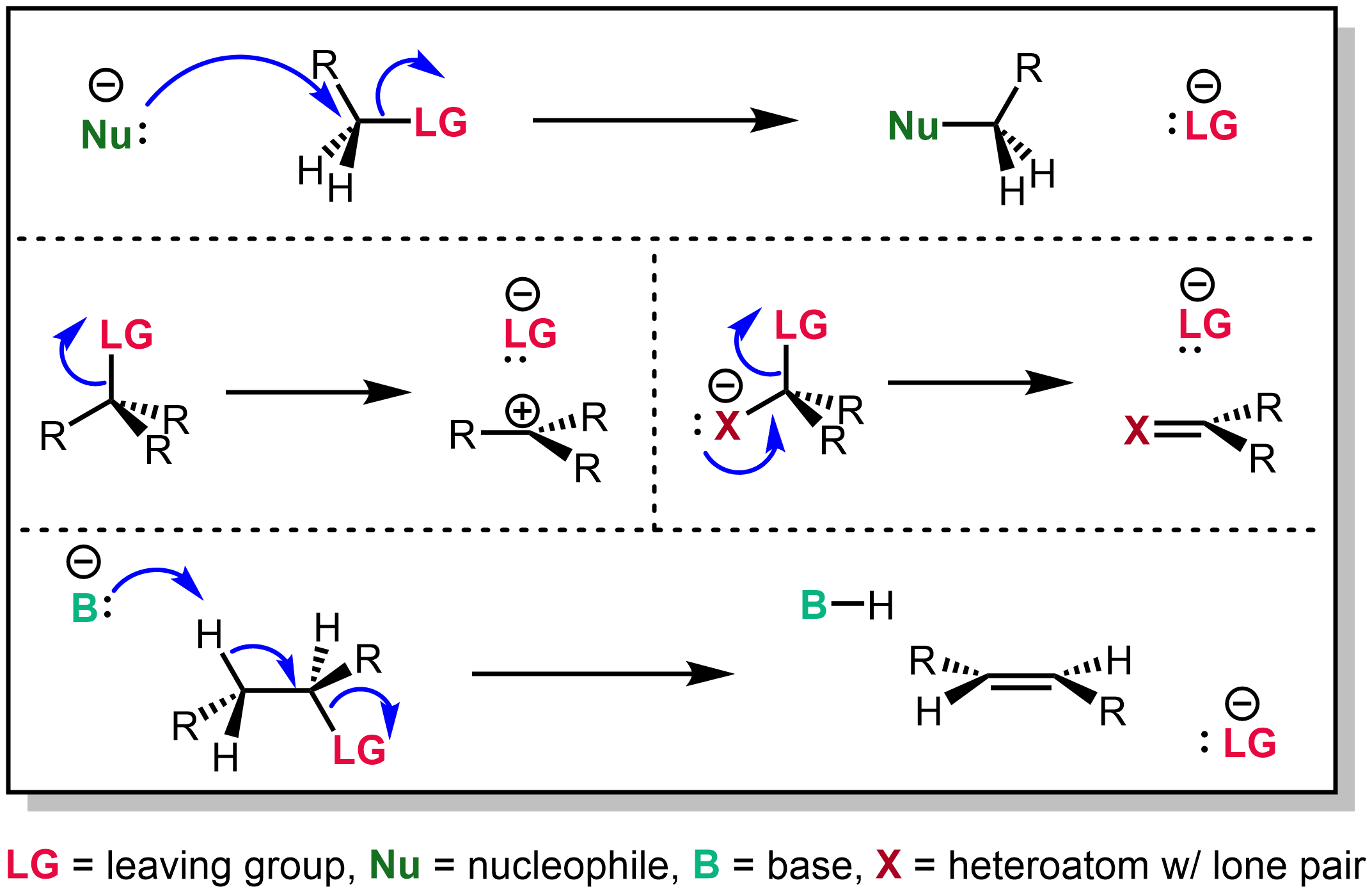 In
In

 Poor leaving groups favor the E1cB mechanism, but as the leaving group improves, transition state ''BC''‡ becomes lower in energy. First, the rate-determining step shifts from leaving-group elimination out of intermediate ''B'' to deprotonation via transition state ''AB''‡ (not pictured). Eventually, ''BC''‡ is no longer stationary on the potential energy surface, and the reaction becomes a concerted E2 elimination (albeit very asynchronous in the diagrammed case).
Poor leaving groups favor the E1cB mechanism, but as the leaving group improves, transition state ''BC''‡ becomes lower in energy. First, the rate-determining step shifts from leaving-group elimination out of intermediate ''B'' to deprotonation via transition state ''AB''‡ (not pictured). Eventually, ''BC''‡ is no longer stationary on the potential energy surface, and the reaction becomes a concerted E2 elimination (albeit very asynchronous in the diagrammed case).
 In Friedel-Crafts alkylations, the normal halogen leaving group order is reversed so that the rate of the reaction follows RF > RCl > RBr > RI. This effect is due to their greater ability to complex the
In Friedel-Crafts alkylations, the normal halogen leaving group order is reversed so that the rate of the reaction follows RF > RCl > RBr > RI. This effect is due to their greater ability to complex the
 In one study, reactivities increased in the order chloride (krel = 1), iodide (krel = 91), tosylate (krel = 3.7), triflate (krel = 1.4), phenyliodonium tetrafluoroborate (, krel = 1.2). In general, leaving groups from dialkylhalonium ions increase in lability as .
In one study, reactivities increased in the order chloride (krel = 1), iodide (krel = 91), tosylate (krel = 3.7), triflate (krel = 1.4), phenyliodonium tetrafluoroborate (, krel = 1.2). In general, leaving groups from dialkylhalonium ions increase in lability as .
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a leaving group typically means a molecular fragment that departs with an electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper ...
during a reaction step
In chemistry, a reaction step of a chemical reaction is defined as: ''"An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate (or, for the first step, the reactants) is converted into the ne ...
with heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge
In chemistry, a nucleofuge () is a leaving group which retains the lone pair of electrons from its previous bond with another species. For example, in the SN2 mechanism, a nucleophile attacks an organic compound containing the nucleofuge (the b ...
''; although IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
gives the term a broader definition.
A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes.
Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
from bond heterolysis. Common anionic leaving groups are , and halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s and sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
esters such as tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
ate (). Water (), alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
(), and amines
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
() are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
(H−) serve as leaving groups only extremely rarely.
Nomenclature
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
defines a leaving group to be any group of atoms that detaches from the main substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
during a reaction step
In chemistry, a reaction step of a chemical reaction is defined as: ''"An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate (or, for the first step, the reactants) is converted into the ne ...
. The term thus includes groups that depart ''without'' an electron pair in a heterolytic cleavage ( electrofuges), like or , which commonly depart in electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
reactions. Similarly, species of high thermodynamic stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; th ...
like nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
() or carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
() commonly act as leaving groups in homolytic bond cleavage reactions of radical species.
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the term leaving group is rarely used for such species, being restricted only to nucleofugal leaving groups. Leaving groups are generally anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s or neutral species, departing from neutral or cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic substrates, respectively, though in rare cases, cations leaving from a dication
A dication is any cation, of general formula X2+, formed by the removal of two electrons from a neutral species.
Diatomic dications corresponding to stable neutral species (e.g. formed by removal of two electrons from H2) often decay quickly int ...
ic substrate are also known.
This article will follow the organic chemistry convention.
Overview
Leaving group ability manifests physically in a fastreaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
. Equivalently, reactions involving good leaving groups have low activation barriers and relatively stable transition states. Because different reaction mechanisms have different transition states, leaving group ability depends on the reaction in question.
For example, consider the first step of an SN1 or E1 reaction in neutral media: ionization, with an anionic leaving group.
Because the leaving group gains negative charge in the transition state (and products), a good leaving group must stabilize this negative charge and form a stable anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
. Strong bases such as and tend to make poor leaving groups, as they cannot stabilize further negative charge; whereas extremely weak bases, such as OSO2CH, leave easily. Mathematically, leaving groups typically exhibit Bell–Evans–Polanyi correlation between the dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (''K''D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex ...
for their conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the rever ...
(p''K''aH) and lability.
Context-dependence
The correlation in SN1 and E1 reactions between leaving group ability and p''K''aH is not perfect. Leaving group ability reflects the energy difference (Δ''G''‡) between neutral starting materials and the partially-charged, partially-bonded transition state. The p''K''aH, however, represents net energy difference (Δ''G°'') for the (possibly multi-step) adduction equilibrium between the leaving group and a solvated proton.Hammond's postulate
Hammond's postulate (or alternatively the Hammond–Leffler postulate), is a hypothesis in physical organic chemistry which describes the geometric structure of the transition state in an organic chemical reaction. First proposed by George Hammo ...
explains conceptually when and why the two energy differences correlate.
For reactions with a different transition state, other aspects of the leaving group may govern. In acid-catalyzed reactions' rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
, only adducts between the formal leaving group and the acid catalyst depart. In those cases, leaving group ability correlates with bond strength to the catalyst (see ). Even more dramatically, benzoate anions decarboxylate when heated with a copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
salt catalyst, in principle expulsing an aryl anion from . The true leaving group is most likely an arylcopper compound rather than the aryl anion salt.
Even for the same reaction mechanism in the same media, relative lability may depend on the other reagents. In the substitutions tabulated below, ethoxide displaces tosylate before any halide, but ''para''-thiocresolate prefers to displace iodide and even bromide before tosylate.
In typical reactions
Many organic chemistry textbooks offer a table comparing typical leaving groups' ability across common reactions: It is exceedingly rare for groups such as (hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
s), ( alkyl anions, R = alkyl or H), or (aryl anions, Ar = aryl) to depart with a pair of electrons because of the high energy of these species. The Chichibabin reaction provides an example of hydride as a leaving group, while the Wolff-Kishner reaction and Haller-Bauer reaction feature unstabilized carbanion leaving groups.
SN2 reactions
For SN2 reactions, typical synthetically-useful leaving groups include , , and . Phosphate and carboxylate substrates are more likely to react by competitive addition-elimination, while sulfonium and ammonium salts generally form ylides or undergo E2 elimination. Phenoxides () constitute the lower limit for feasible SN2 leaving groups: very strong nucleophiles like or demethylateanisole
Anisole, or methoxybenzene, is an organic compound with the formula . It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly ...
derivatives through SN2 displacement at the methyl group. Hydroxide, alkoxides, amides, hydride, and alkyl anions do not serve as leaving groups in SN2 reactions.
In basic eliminations
When anionic or dianionic tetrahedral intermediates collapse, the high electron density of the neighboring heteroatom facilitates the expulsion of even a very poor leaving group. This dramatic departure occurs because forming a very strong C=O double-bond can drive an otherwise unfavorable reaction forward. For example, evenamides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a p ...
expulse R2N−, an extremely poor leaving group, in nucleophilic acyl substitution
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl group ( ...
.
This elimination of poor leaving groups also extends vinylogously to conjugate base eliminations. Many E1cb reactions (e.g. the aldol condensation) commonly involve a hydroxide leaving group from an enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
ate β position.
Likewise, in SNAr reactions, the rate is generally increased when the leaving group is fluoride relative to the other halogens. This effect is due to the fact that the highest energy transition state for this two step addition-elimination process occurs in the first step, where fluoride's greater electron withdrawing capability relative to the other halides stabilizes the developing negative charge on the aromatic ring. The departure of the leaving group takes place quickly from this high energy Meisenheimer complex A Meisenheimer complex or Jackson–Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene compound, arene carrying electron withdrawing groups and a nucleophile. These complexes are found as reactive intermediates in nu ...
, and since the departure is not involved in the rate limiting step, it does not affect the overall rate of the reaction.
E1cb reactions
E1cb reactions proceed with poor leaving groups, but because the C=C double bond is weaker than a C=O bond, the leaving group affects the elimination mechanism.
 Poor leaving groups favor the E1cB mechanism, but as the leaving group improves, transition state ''BC''‡ becomes lower in energy. First, the rate-determining step shifts from leaving-group elimination out of intermediate ''B'' to deprotonation via transition state ''AB''‡ (not pictured). Eventually, ''BC''‡ is no longer stationary on the potential energy surface, and the reaction becomes a concerted E2 elimination (albeit very asynchronous in the diagrammed case).
Poor leaving groups favor the E1cB mechanism, but as the leaving group improves, transition state ''BC''‡ becomes lower in energy. First, the rate-determining step shifts from leaving-group elimination out of intermediate ''B'' to deprotonation via transition state ''AB''‡ (not pictured). Eventually, ''BC''‡ is no longer stationary on the potential energy surface, and the reaction becomes a concerted E2 elimination (albeit very asynchronous in the diagrammed case).
Activation
In SN1 and E1 reactions, protonation or complexation with aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
commonly transform a poor leaving group into a good one. Then the reaction proceeds with (respectively) nucleophilic attack or elimination. It is by protonation before departure that a molecule can formally lose such poor leaving groups as hydroxide.
The same principle is at work in the Friedel-Crafts reaction. Here, a strong Lewis acid is required to generate either a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
from an alkyl halide in the Friedel-Crafts alkylation reaction or an acylium ion from an acyl halide.
 In Friedel-Crafts alkylations, the normal halogen leaving group order is reversed so that the rate of the reaction follows RF > RCl > RBr > RI. This effect is due to their greater ability to complex the
In Friedel-Crafts alkylations, the normal halogen leaving group order is reversed so that the rate of the reaction follows RF > RCl > RBr > RI. This effect is due to their greater ability to complex the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
catalyst, and the actual group that leaves is an "ate" complex between the Lewis acid and the departing leaving group.
"Super" and "hyper" leaving groups
Super leaving groups generally mean any leaving group comparable totriflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
; such compounds generally autoionize if the electrofuge can form a stable carbocation. Thus, the most commonly encountered organic triflates are alkenyl, aryl, and methyl triflates, of which none can form stable carbocations. Conversely, mixed acyl-triflyl anhydrides smoothly undergo Friedel-Crafts acylation, where the corresponding acyl halides would require a strong Lewis acid catalyst.
Even more reactive are the hyper leaving groups, which are stronger than triflate and react with reductive elimination. Prominent hyper leaving groups include various halonium ions, such as diaryl iodonium salts; and other λ3-iodanes.
Hyper leaving groups can be displaced by extraordinarily weak nucleophiles, in part from the entropic favorability of splitting one molecule into three. Heating neat samples of under reduced pressure methylated the very poorly nucleophilic carborane anion, with concomitant expulsion of the leaving group. Likewise, dialkylhalonium hexafluoroantimonate salts alkylate other alkyl halides to give exchanged products.
 In one study, reactivities increased in the order chloride (krel = 1), iodide (krel = 91), tosylate (krel = 3.7), triflate (krel = 1.4), phenyliodonium tetrafluoroborate (, krel = 1.2). In general, leaving groups from dialkylhalonium ions increase in lability as .
In one study, reactivities increased in the order chloride (krel = 1), iodide (krel = 91), tosylate (krel = 3.7), triflate (krel = 1.4), phenyliodonium tetrafluoroborate (, krel = 1.2). In general, leaving groups from dialkylhalonium ions increase in lability as .
See also
*Entering group (a relatively uncommon term) — a species that bonds to a substrate-derived intermediate. * Electrofuge *Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
*Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
*Nucleofuge
In chemistry, a nucleofuge () is a leaving group which retains the lone pair of electrons from its previous bond with another species. For example, in the SN2 mechanism, a nucleophile attacks an organic compound containing the nucleofuge (the b ...
*Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
*Substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
References
{{Authority control Organic reactions Reaction mechanisms