benzene on:
[Wikipedia]
[Google]
[Amazon]
Benzene is an organic
Historic Benzene Formulae Kekulé (original).png, Kekulé's 1872 modification of his 1865 theory, illustrating rapid alternation of double bondsCritics pointed out a problem with Kekulé's original (1865) structure for benzene: Whenever benzene underwent substitution at the ortho position, two distinguishable isomers should have resulted, depending on whether a double bond or a single bond existed between the carbon atoms to which the substituents were attached; however, no such isomers were observed. In 1872, Kekulé suggested that benzene had two complementary structures and that these forms rapidly interconverted, so that if there were a double bond between any pair of carbon atoms at one instant, that double bond would become a single bond at the next instant (and vice versa). To provide a mechanism for the conversion process, Kekulé proposed that the valency of an atom is determined by the frequency with which it collided with its neighbors in a molecule. As the carbon atoms in the benzene ring collided with each other, each carbon atom would collide twice with one neighbor during a given interval and then twice with its other neighbor during the next interval. Thus, a double bond would exist with one neighbor during the first interval and with the other neighbor during the next interval. Therefore, between the carbon atoms of benzene there were no fixed (i.e., constant) and distinct single or double bonds; instead, the bonds between the carbon atoms were identical. Se
pages 86–89
of Auguste Kekulé (1872) "Ueber einige Condensationsprodukte des Aldehyds" (On some condensation products of aldehydes), ''Liebig's Annalen der Chemie und Pharmacie'', 162(1): 77–124, 309–320. From p. 89: ''"Das einfachste Mittel aller Stöße eines Kohlenstoffatoms ergiebt sich aus der Summe der Stöße der beiden ersten Zeiteinheiten, die sich dann periodisch wiederholen. ... man sieht daher, daß jedes Kohlenstoffatom mit den beiden anderen, ... daß diese Verschiedenheit nur eine scheinbare, aber keine wirkliche ist."'' (The simplest average of all the collisions of a carbon atom n benzenecomes from the sum of the collisions during the first two units of time, which then periodically repeat. ... thus one sees that each carbon atom collides equally often with the two others against which it bumps, ndthus stands in exactly the same relation with its two neighbors. The usual structural formula for benzene expresses, of course, only the collisions that occur during ''one'' unit of time, thus during one phase, and so one is led to the view
The new understanding of benzene, and hence of all aromatic compounds, proved to be so important for both pure and applied chemistry that in 1890 the German Chemical Society organized an elaborate appreciation in Kekulé's honor, celebrating the twenty-fifth anniversary of his first benzene paper. Here Kekulé spoke of the creation of the theory. He said that he had discovered the ring shape of the benzene molecule after having a reverie or day-dream of a snake biting its own tail (a symbol in ancient cultures known as the

File:Benzene_uses.png, center, Major commodity chemicals and polymers derived from benzene. Clicking on the image loads the appropriate article, 600px, thumb
rect 39 660 435 807
 The most widely practiced example of this reaction is the ethylation of benzene.
::
The most widely practiced example of this reaction is the ethylation of benzene.
:: Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst. Similarly, the Friedel-Crafts acylation is a related example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong
Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst. Similarly, the Friedel-Crafts acylation is a related example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong 
 Benzene is classified as a
Benzene is classified as a
Benzene
at ''
International Chemical Safety Card 0015
*
Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
Video Recording
of Sir John Cadogan giving a lecture on Benzene at the Royal Institution, 22nd September 1991
Substance profile
NLM Hazardous Substances Databank – Benzene
{{Authority control Annulenes Aromatic hydrocarbons Aromatic solvents Carcinogens Commodity chemicals GABAA receptor positive allosteric modulators Hydrocarbon solvents IARC Group 1 carcinogens Immunotoxins Mutagens Chemical hazards Petrochemicals Simple aromatic rings Six-membered rings Soil contamination Sweet-smelling chemicals Teratogens
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the molecular formula C6H6. The benzene molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
is composed of six carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms joined in a planar hexagonal ring with one hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom attached to each. Because it contains only carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and hydrogen atoms, benzene is classed as a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
.
Benzene is a natural constituent of petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and is one of the elementary petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable s ...
s. Due to the cyclic continuous pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
s between the carbon atoms, benzene is classed as an aromatic hydrocarbon
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
. Benzene is a colorless and highly flammable
A combustible material is a material that can burn (i.e., sustain a flame) in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort ...
liquid with a sweet smell, and is partially responsible for the aroma of gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. They are common and exist in a variety of settings and products, not limited to Indoor mold, house mold, Upholstery, upholstered furnitur ...
.
Benzene is classified as a carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
. Its particular effects on human health
Health has a variety of definitions, which have been used for different purposes over time. In general, it refers to physical and emotional well-being, especially that associated with normal functioning of the human body, absent of disease, pain ...
, such as the long-term results of accidental exposure, have been reported on by news organizations such as ''The New York Times
''The New York Times'' (''NYT'') is an American daily newspaper based in New York City. ''The New York Times'' covers domestic, national, and international news, and publishes opinion pieces, investigative reports, and reviews. As one of ...
''. For instance, a 2022 article stated that benzene contamination in the Boston metropolitan area caused hazardous conditions in multiple places, with the publication noting that the compound may eventually cause leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
in some individuals.
History
Discovery
The word "''benzene''" derives from "''gum benzoin''" ( benzoin resin), an aromatic resin known since ancient times in Southeast Asia, and later to European pharmacists and perfumers in the 16th century via trade routes. Anacid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ic material was derived from benzoin by sublimation, and named "flowers of benzoin", or benzoic acid. The hydrocarbon derived from benzoic acid thus acquired the name benzin, benzol, or benzene. Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name ''bicarburet of hydrogen''. In 1833, Eilhard Mitscherlich produced it by distilling benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
(from gum benzoin) and lime. He gave the compound the name ''benzin''. In 1836, the French chemist Auguste Laurent named the substance "phène"; this word has become the root of the English word "phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
", which is hydroxylated benzene, and " phenyl", the radical formed by abstraction of a hydrogen atom from benzene.
In 1845, Charles Blachford Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoria ...
. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Gradually, the sense developed among chemists that a number of substances were chemically related to benzene, comprising a diverse chemical family. In 1855, Hofmann was the first to apply the word "aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
" to designate this family relationship, after a characteristic property of many of its members. In 1997, benzene was detected in deep space.
Ring formula
The empirical formula for benzene was long known, but its highly polyunsaturated structure, with just onehydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atom for each carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom, was challenging to determine. Archibald Scott Couper in 1858 and Johann Josef Loschmidt in 1861 suggested possible structures that contained multiple double bonds or multiple rings, but in these years very little was known about aromatic chemistry, and so chemists were unable to adduce appropriate evidence to favor any particular formula.
But many chemists had begun to work on aromatic substances, especially in Germany, and relevant data was coming fast. In 1865, the German chemist Friedrich August Kekulé published a paper in French (for he was then teaching in Francophone Belgium) suggesting that the structure contained a ring of six carbon atoms with alternating single and double bonds. The next year he published a much longer paper in German on the same subject. On p. 100, Kekulé suggests that the carbon atoms of benzene could form a "chaîne fermée" (closed chain). Kekulé used evidence that had accumulated in the intervening years—namely, that there always appeared to be only one isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
of any monoderivative of benzene, and that there always appeared to be exactly three isomers of every disubstituted derivative—now understood to correspond to the ortho, meta, and para patterns of arene substitution—to argue in support of his proposed structure. Kekulé's symmetrical ring could explain these curious facts, as well as benzene's 1:1 carbon-hydrogen ratio.
pages 86–89
of Auguste Kekulé (1872) "Ueber einige Condensationsprodukte des Aldehyds" (On some condensation products of aldehydes), ''Liebig's Annalen der Chemie und Pharmacie'', 162(1): 77–124, 309–320. From p. 89: ''"Das einfachste Mittel aller Stöße eines Kohlenstoffatoms ergiebt sich aus der Summe der Stöße der beiden ersten Zeiteinheiten, die sich dann periodisch wiederholen. ... man sieht daher, daß jedes Kohlenstoffatom mit den beiden anderen, ... daß diese Verschiedenheit nur eine scheinbare, aber keine wirkliche ist."'' (The simplest average of all the collisions of a carbon atom n benzenecomes from the sum of the collisions during the first two units of time, which then periodically repeat. ... thus one sees that each carbon atom collides equally often with the two others against which it bumps, ndthus stands in exactly the same relation with its two neighbors. The usual structural formula for benzene expresses, of course, only the collisions that occur during ''one'' unit of time, thus during one phase, and so one is led to the view
hat
A hat is a Headgear, head covering which is worn for various reasons, including protection against weather conditions, ceremonial reasons such as university graduation, religious reasons, safety, or as a fashion accessory. Hats which incorpor ...
doubly substituted derivatives f benzenemust be different at positions 1,2 and 1,6 f the benzene ring If the idea hat wasjust presented—or a similar one—can be regarded as correct, then tfollows therefrom that this difference etween the bonds at positions 1,2 and 1,6is only an apparent ne not a real ne)
ouroboros
The ouroboros or uroboros (; ) is an ancient symbol depicting a serpent symbolism, snake or European dragon, dragon Autocannibalism, eating its own tail. The ouroboros entered Western tradition via Egyptian mythology, ancient Egyptian iconogra ...
). This vision, he said, came to him after years of studying the nature of carbon-carbon bonds. This was seven years after he had solved the problem of how carbon atoms could bond to up to four other atoms at the same time. Curiously, a similar, humorous depiction of benzene had appeared in 1886 in a pamphlet entitled ''Berichte der Durstigen Chemischen Gesellschaft'' (Journal of the Thirsty Chemical Society), a parody of the ''Berichte der Deutschen Chemischen Gesellschaft'', only the parody had monkeys seizing each other in a circle, rather than snakes as in Kekulé's anecdote. Some historians have suggested that the parody was a lampoon of the snake anecdote, possibly already well known through oral transmission even if it had not yet appeared in print. Kekulé's 1890 speech in which this anecdote appeared has been translated into English. If the anecdote is the memory of a real event, circumstances mentioned in the story suggest that it must have happened early in 1862.
In 1929, the cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale using X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
methods. Using large crystals of hexamethylbenzene, a benzene derivative with the same core of six carbon atoms, Lonsdale obtained diffraction patterns. Through calculating more than thirty parameters, Lonsdale demonstrated that the benzene ring could not be anything but a flat hexagon, and provided accurate distances for all carbon-carbon bonds in the molecule.
Nomenclature
The German chemist Wilhelm Körner suggested the prefixes ortho-, meta-, para- to distinguish di-substituted benzene derivatives in 1867; however, he did not use the prefixes to distinguish the relative positions of the substituents on a benzene ring. It was the German chemist Carl Gräbe who, in 1869, first used the prefixes ortho-, meta-, para- to denote specific relative locations of the substituents on a di-substituted aromatic ring (viz, naphthalene). In 1870, the German chemist Viktor Meyer first applied Gräbe's nomenclature to benzene.Early applications
In 1903, Ludwig Roselius popularized the use of benzene to decaffeinatecoffee
Coffee is a beverage brewed from roasted, ground coffee beans. Darkly colored, bitter, and slightly acidic, coffee has a stimulating effect on humans, primarily due to its caffeine content, but decaffeinated coffee is also commercially a ...
. This discovery led to the production of Sanka. This process was later discontinued. Benzene was historically used as a significant component in many consumer products such as liquid wrench, several paint strippers, rubber cements, spot removers, and other products. Manufacture of some of these benzene-containing formulations ceased in about 1950, although Liquid Wrench continued to contain significant amounts of benzene until the late 1970s.
Occurrence
Trace amounts of benzene are found in petroleum and coal. It is a byproduct of the incomplete combustion of many materials. For commercial use, untilWorld War II
World War II or the Second World War (1 September 1939 – 2 September 1945) was a World war, global conflict between two coalitions: the Allies of World War II, Allies and the Axis powers. World War II by country, Nearly all of the wo ...
, much of benzene was obtained as a by-product of coke production (or "coke-oven light oil") for the steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
industry. However, in the 1950s, increased demand for benzene, especially from the growing polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s industry, necessitated the production of benzene from petroleum. Today, most benzene comes from the petrochemical industry
file:Jampilen Petrochemical Co. 02.jpg, 300px, Jampilen Petrochemical co., Asaluyeh, Iran
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics industry, plastics (poly ...
, with only a small fraction being produced from coal. Benzene has been detected on Mars
Mars is the fourth planet from the Sun. It is also known as the "Red Planet", because of its orange-red appearance. Mars is a desert-like rocky planet with a tenuous carbon dioxide () atmosphere. At the average surface level the atmosph ...
.
Structure
X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
shows that all six carbon-carbon bonds in benzene are of the same length, at 140 picometres (pm). The C–C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s are greater than a double bond (135 pm) but shorter than a single bond (147 pm). This intermediate distance is caused by electron delocalization: the electrons for C=C bonding are distributed equally between each of the six carbon atoms. Benzene has 6 hydrogen atoms, fewer than the corresponding parent alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whi ...
, hexane
Hexane () or ''n''-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
Hexane is a colorless liquid, odorless when pure, and with a boiling point of approximately . It is widely used as ...
, which has 14. Benzene and cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
have a similar structure; only the ring of delocalized electrons and the loss of one hydrogen per carbon distinguishes it from cyclohexane. The molecule is planar. The molecular orbital description involves the formation of three delocalized π orbitals spanning all six carbon atoms, while the valence bond description involves a superposition of resonance structures. It is likely that this stability contributes to the peculiar molecular and chemical properties known as aromaticity. To reflect the delocalized nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms.
Derivatives of benzene occur sufficiently often as a component of organic molecules, so much so that the Unicode
Unicode or ''The Unicode Standard'' or TUS is a character encoding standard maintained by the Unicode Consortium designed to support the use of text in all of the world's writing systems that can be digitized. Version 16.0 defines 154,998 Char ...
Consortium has allocated a symbol in the Miscellaneous Technical
Miscellaneous Technical is a Unicode block ranging from U+2300 to U+23FF. It contains various common symbols which are related to and used in the various technical, programming language, and academic professions. For example:
* Symbol ⌂ (HTML ...
block with the code U+232C (⌬) to represent it with three double bonds, and U+23E3 (⏣) for a delocalized version.
Benzene derivatives
Many important chemical compounds are derived from benzene by replacing one or more of its hydrogen atoms with anotherfunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
. Examples of simple benzene derivatives are phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
, and aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
, abbreviated PhOH, PhMe, and PhNH2, respectively. Linking benzene rings gives biphenyl, C6H5–C6H5. Further loss of hydrogen gives "fused" aromatic hydrocarbons, such as naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
, anthracene, phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, expl ...
, and pyrene. The limit of the fusion process is the hydrogen-free allotrope of carbon, graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
.
In heterocycles, carbon atoms in the benzene ring are replaced with other elements. The most important variations contain nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. Replacing one CH with N gives the compound pyridine, C5H5N. Although benzene and pyridine are ''structurally'' related, benzene cannot be converted into pyridine. Replacement of a second CH bond with N gives, depending on the location of the second N, pyridazine, pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The oth ...
, or pyrazine.
Production
Four chemical processes contribute to industrial benzene production:catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum naphtha, naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydr ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
hydrodealkylation, toluene disproportionation, and steam cracking etc. According to the ATSDR Toxicological Profile for benzene, between 1978 and 1981, catalytic reformates accounted for approximately 44–50% of the total U.S. benzene production.
Catalytic reforming
In catalytic reforming, a mixture ofhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s with boiling points between 60 and 200 °C is blended with hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas and then exposed to a bifunctional platinum chloride or rhenium chloride catalyst at 500–525 °C and pressures ranging from 8–50 atm. Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products of the reaction are then separated from the reaction mixture (or reformate) by extraction with any one of a number of solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s, including diethylene glycol or sulfolane, and benzene is then separated from the other aromatics by distillation. The extraction step of aromatics from the reformate is designed to produce aromatics with lowest non-aromatic components. Recovery of the aromatics, commonly referred to as BTX (benzene, toluene and xylene isomers), involves such extraction and distillation steps.
In similar fashion to this catalytic reforming, UOP and BP commercialized a method from LPG (mainly propane and butane) to aromatics.
Toluene hydrodealkylation
Toluene hydrodealkylation convertstoluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
to benzene. In this hydrogen-intensive process, toluene is mixed with hydrogen, then passed over a chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
, molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
, or platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
catalyst at 500–650 °C and 20–60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst (at the similar reaction condition). Under these conditions, toluene undergoes dealkylation to benzene and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
:
:
This irreversible reaction is accompanied by an equilibrium side reaction that produces
biphenyl (diphenyl) at higher temperature:
:2 +
If the raw material stream contains much non-aromatic components (paraffins or naphthenes), those are likely decomposed to lower hydrocarbons such as methane, which increases the consumption of hydrogen.
A typical reaction yield exceeds 95%. Sometimes, xylenes and heavier aromatics are used in place of toluene, with similar efficiency.
This is often called "on-purpose" methodology to produce benzene, compared to conventional BTX (benzene-toluene-xylene) extraction processes.
Toluene disproportionation
Toluene disproportionation (TDP) is the conversion of toluene to benzene and xylene. Given that demand for ''para''-xylene ( ''p''-xylene) substantially exceeds demand for other xylene isomers, a refinement of the TDP process called Selective TDP (STDP) may be used. In this process, the xylene stream exiting the TDP unit is approximately 90% ''p''-xylene. In some systems, even the benzene-to-xylenes ratio is modified to favor xylenes.Steam cracking
Steam cracking is the process for producingethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
and other alkenes from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid by-product called '' pyrolysis gasoline''. Pyrolysis gasoline can be blended with other hydrocarbons as a gasoline additive, or routed through an extraction process to recover BTX aromatics (benzene, toluene and xylenes).
Other methods
Although of no commercial significance, many other routes to benzene exist.Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
and halobenzenes can be reduced with metals. Benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
and its salts undergo decarboxylation to benzene. The reaction of the diazonium compound
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compou ...
derived from aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
with hypophosphorus acid gives benzene. Alkyne trimerisation of acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
gives benzene. Complete decarboxylation of mellitic acid gives benzene.
Uses
Benzene is used mainly as an intermediate to make other chemicals, above all ethylbenzene (and otheralkylbenzenes
An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains. Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by Alkyl group, alkyl ...
), cumene, cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
, and nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced ...
. In 1988 it was reported that two-thirds of all chemicals on the American Chemical Society
The American Chemical Society (ACS) is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 155,000 members at all ...
's lists contained at least one benzene ring. More than half of the entire benzene production is processed into ethylbenzene, a precursor to styrene, which is used to make polymers and plastics like polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
. Some 20% of the benzene production is used to manufacture cumene, which is needed to produce phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
and acetone for resins and adhesives. Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
consumes around 10% of the world's benzene production; it is primarily used in the manufacture of nylon fibers, which are processed into textiles and engineering plastics. Smaller amounts of benzene are used to make some types of rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
s, lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, ...
s, dyes, detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
s, drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
s, explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An ex ...
s, and pesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s. In 2013, the biggest consumer country of benzene was China, followed by the USA. Benzene production is currently expanding in the Middle East and in Africa, whereas production capacities in Western Europe and North America are stagnating.
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
is now often used as a substitute for benzene, for instance as a fuel additive. The solvent-properties of the two are similar, but toluene is less toxic and has a wider liquid range. Toluene is also processed into benzene.
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
rect 665 60 1062 207 Ethylbenzene
rect 665 426 1062 579 Cumene
rect 665 795 1062 942 Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
rect 665 1161 1062 1317 Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
rect 665 1533 1062 1686 Chlorobenzene
rect 1215 345 1614 495 Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
rect 1215 636 1614 783 Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
rect 1764 57 2163 210 Styrene
rect 1764 432 2163 585 Bisphenol A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is Solubility, soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on a ...
rect 1764 1083 2163 1233 Adipic acid
rect 1764 1332 2163 1482 Caprolactam
rect 2313 57 2712 207 Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
rect 2313 315 2712 462 Polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate ester, carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, toughness, tough materials, and some grades are optically transp ...
rect 2313 570 2712 717 Epoxy resin
rect 2313 822 2712 975 Phenolic resin
rect 2313 1083 2712 1233 Nylon 6-6
rect 2313 1335 2712 1485 Nylon 6
desc bottom-left
Component of gasoline
As agasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When for ...
(petrol) additive, benzene increases the octane rating
An octane rating, or octane number, is a standard measure of a liquid fuel, fuel's ability to withstand Compression ratio, compression in an internal combustion engine without causing engine knocking. The higher the octane number, the more compres ...
and reduces knocking. As a consequence, gasoline often contained several percent benzene before the 1950s, when tetraethyl lead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb( C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline beginning in the 192 ...
replaced it as the most widely used antiknock additive. With the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some nations. In the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
, concern over its negative health effects and the possibility of benzene entering the groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
has led to stringent regulation of gasoline's benzene content, with limits typically around 1%. European petrol specifications now contain the same 1% limit on benzene content. The United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent agency of the United States government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it began operation on De ...
introduced new regulations in 2011 that lowered the benzene content in gasoline to 0.62%.
In some European languages, the word for petroleum or gasoline is an exact cognate of "benzene". For instance all Scandinavian languages refer to gasoline as 'bensin'. In Catalan the word 'benzina' can be used for gasoline, though now it is relatively rare.
Reactions
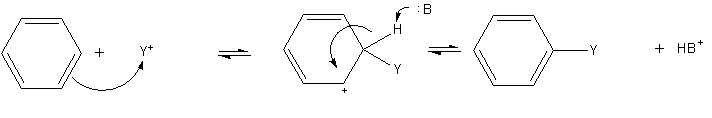
The most common reactions of benzene involve substitution of a proton by other groups. Electrophilic aromatic substitution is a general method of derivatizing benzene. Benzene is sufficiently nucleophilic that it undergoes substitution by acylium ions and alkyl carbocations to give substituted derivatives. : The most widely practiced example of this reaction is the ethylation of benzene.
::
The most widely practiced example of this reaction is the ethylation of benzene.
:: Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst. Similarly, the Friedel-Crafts acylation is a related example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong
Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst. Similarly, the Friedel-Crafts acylation is a related example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
catalyst such as aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
or Iron(III) chloride.
Sulfonation, chlorination, nitration
Using electrophilic aromatic substitution, many functional groups are introduced onto the benzene framework. Sulfonation of benzene involves the use ofoleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid).
Ol ...
, a mixture of sulfuric acid with sulfur trioxide. Sulfonated benzene derivatives are useful detergent
A detergent is a surfactant or a mixture of surfactants with Cleanliness, cleansing properties when in Concentration, dilute Solution (chemistry), solutions. There are a large variety of detergents. A common family is the alkylbenzene sulfonate ...
s. In nitration, benzene reacts with nitronium ions (NO2+), which is a strong electrophile produced by combining sulfuric and nitric acids. Nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced ...
is the precursor to aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
. Chlorination is achieved with chlorine to give chlorobenzene in the presence of a Lewis acid catalyst such as aluminium tri-chloride.
Hydrogenation
Viahydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, benzene and its derivatives convert to cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
and derivatives. This reaction is achieved by the use of high pressures of hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
in the presence of heterogeneous catalysts, such as finely divided nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
. Whereas alkenes can be hydrogenated near room temperatures, benzene and related compounds are more reluctant substrates, requiring temperatures >100 °C. This reaction is practiced on a large scale industrially. In the absence of the catalyst, benzene is impervious to hydrogen. Hydrogenation cannot be stopped to give cyclohexene or cyclohexadienes as these are superior substrates. Birch reduction, a non catalytic process, however selectively hydrogenates benzene to the diene.
Metal complexes
Benzene is an excellentligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
in the organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
chemistry of low-valent metals. Important examples include the sandwich and half-sandwich complexes, respectively, Cr(C6H6)2 and uCl2(C6H6)sub>2.
Health effects
carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
, which increases the risk of cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
and other illnesses, and is also a notorious cause of bone marrow failure. Substantial quantities of epidemiologic, clinical, and laboratory data link benzene to aplastic anemia, acute leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
, bone marrow abnormalities and cardiovascular disease. The specific hematologic malignancies that benzene is associated with include: acute myeloid leukemia (AML), aplastic anemia, myelodysplastic syndrome (MDS), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML).
Carcinogenic activity of benzene was discovered by Swedish pharmacologist in 1897 on female workers of a tire-making factory. The American Petroleum Institute
The American Petroleum Institute (API) is the largest U.S. trade association for the oil and natural gas industry. It claims to represent nearly 600 corporations involved in extraction of petroleum, production, oil refinery, refinement, pipeline ...
(API) stated in 1948 that "it is generally considered that the only absolutely safe concentration for benzene is zero". There is no safe exposure level; even tiny amounts can cause harm. The US Department of Health and Human Services (DHHS) classifies benzene as a human carcinogen. Long-term exposure to excessive levels of benzene in the air causes leukemia, a potentially fatal cancer of the blood-forming organs. In particular, acute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with haematopoiesis, normal blood cell production. Sympt ...
or acute nonlymphocytic leukemia (AML & ANLL) is caused by benzene. IARC rated benzene as "known to be carcinogenic to humans" ( Group 1).
As benzene is ubiquitous in gasoline and hydrocarbon fuels that are in use everywhere, human exposure to benzene is a global health problem. Benzene targets the liver, kidney, lung, heart and brain and can cause DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
strand breaks and chromosomal damage, hence is teratogenic and mutagenic. Benzene causes cancer in animals including humans. Benzene has been shown to cause cancer in both sexes of multiple species of laboratory animals exposed via various routes.
Exposure to benzene
According to the Agency for Toxic Substances and Disease Registry (ATSDR) (2007), benzene is both a synthetically made and naturally occurring chemical from processes that include: volcanic eruptions, wild fires, synthesis of chemicals such asphenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
, production of synthetic fiber
Synthetic fibers or synthetic fibres (in British English; see spelling differences) are fibers made by humans through chemical synthesis, as opposed to natural fibers that are directly derived from living organisms, such as plants like cott ...
s, and fabrication of rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
s, lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, ...
s, pesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s, medications, and dyes. The major sources of benzene exposure are tobacco
Tobacco is the common name of several plants in the genus '' Nicotiana'' of the family Solanaceae, and the general term for any product prepared from the cured leaves of these plants. More than 70 species of tobacco are known, but the ...
smoke, automobile service stations, exhaust from motor vehicles, and industrial emissions; however, ingestion and dermal absorption of benzene can also occur through contact with contaminated water. Benzene is hepatically metabolized and excreted in the urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
. Measurement of air and water levels of benzene is accomplished through collection via activated charcoal tubes, which are then analyzed with a gas chromatograph. The measurement of benzene in humans can be accomplished via urine
Urine is a liquid by-product of metabolism in humans and many other animals. In placental mammals, urine flows from the Kidney (vertebrates), kidneys through the ureters to the urinary bladder and exits the urethra through the penile meatus (mal ...
, blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.
Blood is com ...
, and breath tests; however, all of these have their limitations because benzene is rapidly metabolized in the human body.
Exposure to benzene may lead progressively to aplastic anemia
Anemia (also spelt anaemia in British English) is a blood disorder in which the blood has a reduced ability to carry oxygen. This can be due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin availabl ...
, leukaemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
, and multiple myeloma
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibody, antibodies. Often, no symptoms are noticed initially. As it progresses, bone ...
.
OSHA regulates levels of benzene in the workplace. The maximum allowable amount of benzene in workroom air during an 8-hour workday, 40-hour workweek is 1 ppm. As benzene can cause cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the recommended (8-hour) exposure limit of 0.1 ppm.
Benzene exposure limits
TheUnited States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent agency of the United States government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it began operation on De ...
has set a maximum contaminant level for benzene in drinking water
Drinking water or potable water is water that is safe for ingestion, either when drunk directly in liquid form or consumed indirectly through food preparation. It is often (but not always) supplied through taps, in which case it is also calle ...
at 0.005 mg/L (5 ppb), as promulgated via the U.S. National Primary Drinking Water Regulations. This regulation is based on preventing benzene leukemogenesis. The maximum contaminant level goal ( MCLG), a nonenforceable health goal that would allow an adequate margin of safety for the prevention of adverse effects, is zero benzene concentration in drinking water. The EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene be reported.
The U.S. Occupational Safety and Health Administration
The Occupational Safety and Health Administration (OSHA; ) is a regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. The United States Congress established ...
(OSHA) has set a permissible exposure limit of 1 part of benzene per million parts of air (1 ppm) in the workplace during an 8-hour workday, 40-hour workweek. The short term exposure limit for airborne benzene is 5 ppm for 15 minutes. These legal limits were based on studies demonstrating compelling evidence of health risk to workers exposed to benzene. The risk from exposure to 1 ppm for a working lifetime has been estimated as 5 excess leukemia deaths per 1,000 employees exposed. (This estimate assumes no threshold for benzene's carcinogenic effects.) OSHA has also established an action level of 0.5 ppm to encourage even lower exposures in the workplace.
The U.S. National Institute for Occupational Safety and Health (NIOSH) revised the Immediately Dangerous to Life and Health (IDLH) concentration for benzene to 500 ppm. The current NIOSH definition for an IDLH condition, as given in the NIOSH Respirator Selection Logic, is one that poses a threat of exposure to airborne contaminants when that exposure is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment. The purpose of establishing an IDLH value is (1) to ensure that the worker can escape from a given contaminated environment in the event of failure of the respiratory protection equipment and (2) is considered a maximum level above which only a highly reliable breathing apparatus
A breathing apparatus or breathing set is equipment which allows a person to breathe in a hostile environment where breathing would otherwise be impossible, difficult, harmful, or hazardous, or assists a person to breathe. A respirator, medical v ...
providing maximum worker protection is permitted. publication No. 2005-100. In September 1995, NIOSH issued a new policy for developing recommended exposure limits (RELs) for substances, including carcinogens. As benzene can cause cancer, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the REL (10-hour) of 0.1 ppm. The NIOSH short-term exposure limit (STEL – 15 min) is 1 ppm.
American Conference of Governmental Industrial Hygienists (ACGIH) adopted Threshold Limit Values (TLVs) for benzene at 0.5 ppm TWA and 2.5 ppm STEL.
Toxicology
Biomarkers of exposure
Several tests can determine exposure to benzene. Benzene itself can be measured in breath, blood or urine, but such testing is usually limited to the first 24 hours post-exposure due to the relatively rapid removal of the chemical by exhalation or biotransformation. Most people in developed countries have measureable baseline levels of benzene and other aromatic petroleum hydrocarbons in their blood. In the body, benzene is enzymatically converted to a series of oxidation products including muconic acid, phenylmercapturic acid,phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
, catechol, hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para' ...
and 1,2,4-trihydroxybenzene. Most of these metabolites have some value as biomarkers of human exposure, since they accumulate in the urine in proportion to the extent and duration of exposure, and they may still be present for some days after exposure has ceased. The current ACGIH biological exposure limits for occupational exposure are 500 μg/g creatinine for muconic acid and 25 μg/g creatinine for phenylmercapturic acid in an end-of-shift urine specimen.
Biotransformations
Even if it is not a common substrate for metabolism, benzene can be oxidized by bothbacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
and eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s. In bacteria, dioxygenase enzyme can add an oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
to the ring, and the unstable product is immediately reduced (by NADH) to a cyclic diol with two double bonds, breaking the aromaticity. Next, the diol is newly reduced by NADH to catechol. The catechol is then metabolized to acetyl CoA and succinyl CoA, used by organisms mainly in the citric acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-Co ...
for energy production.
The pathway for the metabolism of benzene is complex and begins in the liver. Several enzymes are involved. These include cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for examp ...
2E1 (CYP2E1), quinine oxidoreductase (NQ01 or DT-diaphorase or NAD(P)H dehydrogenase (quinone 1)), GSH, and myeloperoxidase (MPO). CYP2E1 is involved at multiple steps: converting benzene to oxepin (benzene oxide), phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
to hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para' ...
, and hydroquinone to both benzenetriol and catechol. Hydroquinone, benzenetriol and catechol are converted to polyphenols. In the bone marrow, MPO converts these polyphenols to benzoquinones. These intermediates and metabolites induce genotoxicity by multiple mechanisms including inhibition of topoisomerase II
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of Adenosine triphosphate, ATP, unlike Type I topoisomerase. In this process, t ...
(which maintains chromosome structure), disruption of microtubules (which maintains cellular structure and organization), generation of oxygen free radicals (unstable species) that may lead to point mutations, increasing oxidative stress, inducing DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
strand breaks, and altering DNA methylation (which can affect gene expression). NQ01 and GSH shift metabolism away from toxicity. NQ01 metabolizes benzoquinone toward polyphenols (counteracting the effect of MPO). GSH is involved with the formation of phenylmercapturic acid.
Genetic polymorphisms in these enzymes may induce loss of function or gain of function. For example, mutations in CYP2E1 increase activity and result in increased generation of toxic metabolites. NQ01 mutations result in loss of function and may result in decreased detoxification. Myeloperoxidase mutations result in loss of function and may result in decreased generation of toxic metabolites. GSH mutations or deletions result in loss of function and result in decreased detoxification. These genes may be targets for genetic screening for susceptibility to benzene toxicity.
Molecular toxicology
The paradigm of toxicological assessment of benzene is shifting towards the domain of molecular toxicology as it allows understanding of fundamental biological mechanisms in a better way. Glutathione seems to play an important role by protecting against benzene-induced DNA breaks and it is being identified as a new biomarker for exposure and effect. Benzene causes chromosomal aberrations in the peripheral blood leukocytes and bone marrow explaining the higher incidence of leukemia and multiple myeloma caused by chronic exposure. These aberrations can be monitored using fluorescent in situ hybridization (FISH) with DNA probes to assess the effects of benzene along with the hematological tests as markers of hematotoxicity. Benzene metabolism involves enzymes coded for by polymorphic genes. Studies have shown that genotype at these loci may influence susceptibility to the toxic effects of benzene exposure. Individuals carrying variant of NAD(P)H:quinone oxidoreductase 1 (NQO1), microsomal epoxide hydrolase (EPHX) and deletion of the glutathione S-transferase T1 (GSTT1) showed a greater frequency of DNA single-stranded breaks.Biological oxidation and carcinogenic activity
One way of understanding the carcinogenic effects of benzene is by examining the products of biological oxidation. Pure benzene, for example, oxidizes in the body to produce an epoxide, benzene oxide, which is not excreted readily and can interact with DNA to produce harmful mutations.Routes of exposure
Inhalation
Outdoor air may contain low levels of benzene from automobile service stations, wood smoke, tobacco smoke, the transfer of gasoline, exhaust from motor vehicles, and industrial emissions. About 50% of the entire nationwide (United States) exposure to benzene results from smoking tobacco or from exposure to tobacco smoke. After smoking 32 cigarettes per day, the smoker would take in about 1.8 milligrams (mg) of benzene. This amount is about 10 times the average daily intake of benzene by nonsmokers. Inhaled benzene is primarily expelled unchanged through exhalation. In a human study 16.4 to 41.6% of retained benzene was eliminated through the lungs within five to seven hours after a two- to three-hour exposure to 47 to 110 ppm and only 0.07 to 0.2% of the remaining benzene was excreted unchanged in the urine. After exposure to 63 to 405 mg/m3 of benzene for 1 to 5 hours, 51 to 87% was excreted in the urine as phenol over a period of 23 to 50 hours. In another human study, 30% of absorbed dermally applied benzene, which is primarily metabolized in the liver, was excreted as phenol in the urine.Exposure from soft drinks
Under specific conditions and in the presence of other chemicalsbenzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
(a preservative) and ascorbic acid
Ascorbic acid is an organic compound with formula , originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.
Asco ...
(Vitamin C) may interact to produce benzene. In March 2006, the official Food Standards Agency in United Kingdom
The United Kingdom of Great Britain and Northern Ireland, commonly known as the United Kingdom (UK) or Britain, is a country in Northwestern Europe, off the coast of European mainland, the continental mainland. It comprises England, Scotlan ...
conducted a survey of 150 brands of soft drinks. It found that four contained benzene levels above World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Gen ...
limits. The affected batches were removed from sale. Similar problems were reported by the FDA in the United States.
Contamination of water supply
In 2005, the water supply to the city ofHarbin
Harbin, ; zh, , s=哈尔滨, t=哈爾濱, p=Hā'ěrbīn; IPA: . is the capital of Heilongjiang, China. It is the largest city of Heilongjiang, as well as being the city with the second-largest urban area, urban population (after Shenyang, Lia ...
in China with a population of almost nine million people, was cut off because of a major benzene exposure. Benzene leaked into the Songhua River, which supplies drinking water to the city, after an explosion at a China National Petroleum Corporation (CNPC) factory in the city of Jilin on 13 November 2005.
When plastic water pipes are subject to high heat, the water may be contaminated with benzene.
Genocide
Nazi Germany
Nazi Germany, officially known as the German Reich and later the Greater German Reich, was the German Reich, German state between 1933 and 1945, when Adolf Hitler and the Nazi Party controlled the country, transforming it into a Totalit ...
used benzene administered via injection as one of their many methods for killing.
See also
* BTEX * 1,2,3-Cyclohexatriene * '' Industrial Union Department v. American Petroleum Institute'' * Six-membered aromatic rings with one carbon replaced by another element: borabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyryliumExplanatory notes
References
External links
Benzene
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
International Chemical Safety Card 0015
*
Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
Video Recording
of Sir John Cadogan giving a lecture on Benzene at the Royal Institution, 22nd September 1991
Substance profile
NLM Hazardous Substances Databank – Benzene
{{Authority control Annulenes Aromatic hydrocarbons Aromatic solvents Carcinogens Commodity chemicals GABAA receptor positive allosteric modulators Hydrocarbon solvents IARC Group 1 carcinogens Immunotoxins Mutagens Chemical hazards Petrochemicals Simple aromatic rings Six-membered rings Soil contamination Sweet-smelling chemicals Teratogens