Silicene on:
[Wikipedia]
[Google]
[Amazon]
 Silicene is a two-dimensional
Silicene is a two-dimensional

 Silicene is a two-dimensional
Silicene is a two-dimensional allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
of silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, with a hexagonal honeycomb structure similar to that of graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide.
History
Although theorists had speculated about the existence and possible properties of free-standing silicene, researchers first observed silicon structures that were suggestive of silicene in 2010. Using ascanning tunneling microscope
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
they studied self-assembled silicene nanoribbons and silicene sheets deposited onto a silver crystal, Ag(110) and Ag(111), with atomic resolution. The images revealed hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is de ...
s in a honeycomb structure similar to that of graphene, which, however, were shown to originate from the silver surface mimicking the hexagons. Density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) calculations showed that silicon atoms tend to form such honeycomb structures on silver, and adopt a slight curvature that makes the graphene-like configuration more likely. However, such a model has been invalidated for Si/Ag(110): the Ag surface displays a missing-row reconstruction upon Si adsorption and the honeycomb structures observed are tip artifacts.
This was followed in 2013 by the discovery of dumbbell reconstruction in silicene that explains the formation mechanisms of layered silicene and silicene on Ag.
In 2015, a silicene field-effect transistor was tested. that opens up opportunities for two-dimensional silicon for fundamental science studies and electronic applications.
In 2022, it was found that silicene/Ag(111) growth on top of a Si/Ag(111) surface alloy, functions as a foundation and scaffold for the two-dimensional layer. This, however, raises questions of whether silicene can be truly regarded as two-dimensional material at all, due to its strong chemical bonds to the surface alloy.
Similarities and differences with graphene
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
are similar atoms. They lie above and below each other in the same group on the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, and both have an s2 p2 electronic structure. The 2D structures of silicene and graphene also are quite similar, but they have important differences. While both form hexagonal structures, graphene is completely flat, while silicene forms a buckled hexagonal shape. Its buckled structure gives silicene a tuneable band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
by applying an external electric field. Silicene's hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
reaction is more exothermic than graphene's. Another difference is that since silicon's covalent bonds
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
do not have pi-stacking, silicene does not cluster into a graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
-like form. The formation of a buckled structure in silicene unlike planar structure of graphene has been attributed to strong Pseudo Jahn–Teller distortions arising due to vibronic coupling between closely spaced filled and empty electronic states.
Silicene and graphene have similar electronic structures. Both have a Dirac cone and linear electronic dispersion around the Dirac points. Both also have a quantum spin Hall effect. Both are expected to have the characteristics of massless Dirac fermions that carry charge, but this is only predicted for silicene and has not been observed, likely because it is expected to only occur with free-standing silicene which has not been synthesized. It is believed that the substrate on which the silicene is made has a substantial effect on its electronic properties.
Unlike carbon atoms in graphene, silicon atoms tend to adopt ''sp''3 hybridization over ''sp''2 in silicene, which makes it highly chemically active on the surface and allows its electronic states to be easily tuned by chemical functionalization.
Compared with graphene, silicene has several prominent advantages: (1) a much stronger spin–orbit coupling, which may lead to a realization of quantum spin Hall effect in the experimentally accessible temperature, (2) a better tunability of the band gap, which is necessary for an effective field effect transistor (FET) operating at room temperature, (3) an easier valley polarization and more suitability for valleytronics study.
Unlike graphene, it has been shown that, at least silicene supported by Ag(111) grows on a surface alloy. Hence, decoupling silicene is much less trivial, if possible at all, than decoupling graphene.
Surface alloying
Silicene on Ag(111) grows on top of a Si/Ag(111) surface alloy, which has been shown by a combination of different measurement techniques. The surface alloy precedes the growth of silicene, acting both as foundation and as scaffold for the two-dimensional layer. Upon further increase of silicon coverage, the alloy is covered by silicene, yet pervasivley exists for all coverages. This implies that the properties of the layer are strongly influenced by its alloy.Band gap
Early studies of silicene showed that differentdopants
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optical properties. The amount of dopant is typically very low compared to the material being do ...
within the silicene structure provide the ability to tune its band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
. Very recently, the band gap in epitaxial silicene has been tuned by oxygen adatoms from zero-gap-type to semiconductor-type. With a tunable band gap, specific electronic components could be made-to-order for applications that require specific band gaps. The band gap can be brought down to 0.1 eV, which is considerably smaller than the band gap (0.4 eV) found in traditional field effect transistor
The field-effect transistor (FET) is a type of transistor that uses an electric field to control the current through a semiconductor. It comes in two types: junction FET (JFET) and metal-oxide-semiconductor FET (MOSFET). FETs have three termi ...
s (FETs).
Inducing n-type doping within silicene requires an alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
dopant. Varying the amount adjusts the band gap. Maximum doping increases the band gap 0.5eV. Due to heavy doping, the supply voltage must also be c. 30V. Alkali metal-doped silicene can only produce n-type semiconductors
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels ...
; modern day electronics require a complementary n-type and p-type junction. Neutral doping (i-type) is required to produce devices such as light emitting diodes ( LEDs). LEDs use a p-i-n junction to produce light. A separate dopant must be introduced to generate p-type doped silicene. Iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
(Ir) doped silicene allows p-type silicene to be created. Through platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
(Pt) doping, i-type silicene is possible. With the combination of n-type, p-type and i-type doped structures, silicene has opportunities for use in electronics.
Power dissipation within traditional metal oxide semiconductor field effect transistors (MOSFETs
upright=1.3, Two power MOSFETs in amperes">A in the ''on'' state, dissipating up to about 100 watt">W and controlling a load of over 2000 W. A matchstick is pictured for scale.
In electronics, the metal–oxide–semiconductor field- ...
) generates a bottleneck when dealing with nano-electronics. Tunnel field-effect transistor
The tunnel field-effect transistor (TFET) is an experimental type of transistor. Even though its structure is very similar to a metal–oxide–semiconductor field-effect transistor (MOSFET), the fundamental switching mechanism differs, making this ...
s (TFETs) may become an alternative to traditional MOSFETs because they can have a smaller subthreshold slope
The subthreshold slope is a feature of a MOSFET's current–voltage characteristic.
In the subthreshold region, the drain current behaviour—though being controlled by the gate
A gate or gateway is a point of entry to or from a space en ...
and supply voltage, which reduce power dissipation. Computational studies showed that silicene based TFETs outperform traditional silicon based MOSFETs. Silicene TFETs have an on-state current over 1mA/μm, a sub-threshold slope of 77 mV/decade and a supply voltage of 1.7 V. With this much increased on-state current and reduced supply voltage, power dissipation within these devices is far below that of traditional MOSFETs and its peer TFETs.
Properties
2D silicene is not fully planar, apparently featuring chair-like puckering distortions in the rings. This leads to ordered surface ripples. Hydrogenation of silicenes to silicanes isexothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
. This led to the prediction that the process of conversion of silicene to silicane (hydrogenated silicene) is a candidate for hydrogen storage
Several methods exist for storing hydrogen. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by variou ...
. Unlike graphite, which consists of weakly held stacks of graphene layers through dispersion forces, interlayer coupling in silicenes is very strong.
The buckling of the hexagonal structure of silicene is caused by pseudo Jahn–Teller distortion (PJT). This is caused by strong vibronic coupling of unoccupied molecular orbitals (UMO) and occupied molecular orbitals (OMO). These orbitals are close enough in energy to cause the distortion to high symmetry configurations of silicene. The buckled structure can be flattened by suppressing the PJT distortion by increasing the energy gap between the UMO and OMO. This can be done by adding a lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
ion.
In addition to its potential compatibility with existing semiconductor techniques, silicene has the advantage that its edges do not exhibit oxygen reactivity.
In 2012, several groups independently reported ordered phases on the Ag(111) surface. Results from scanning tunneling spectroscopy
Scanning tunneling spectroscopy (STS), an extension of scanning tunneling microscopy (STM), is used to provide information about the density of electrons in a sample as a function of their energy.
In scanning tunneling microscopy, a metal tip i ...
measurements and from angle-resolved photoemission spectroscopy
Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoel ...
(ARPES) appeared to show that silicene would have similar electronic properties as graphene, namely an electronic dispersion resembling that of relativistic Dirac fermions at the K points of the Brillouin zone
In mathematics and solid state physics, the first Brillouin zone (named after Léon Brillouin) is a uniquely defined primitive cell in reciprocal space
Reciprocal lattice is a concept associated with solids with translational symmetry whic ...
, but the interpretation was later disputed and shown to arise due to a substrate band. A band unfolding technique was used to interpret the ARPES results, revealing the substrate origin of the observed linear dispersion.
Besides silver, silicene has been reported to grow on , and iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
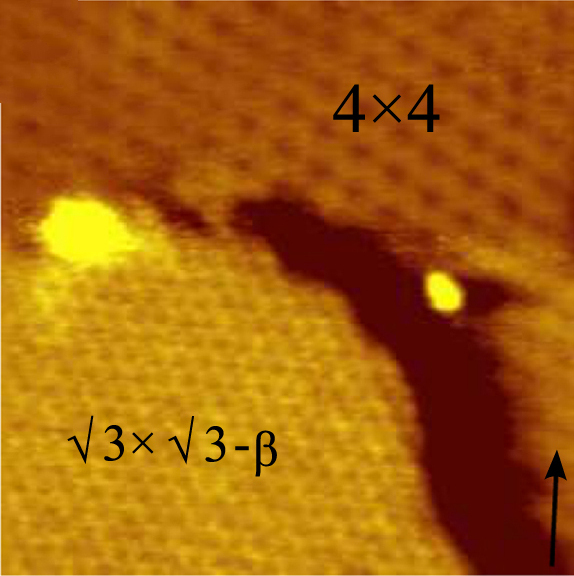
. Theoretical studies predicted that silicene is stable on the Al(111) surface as a honeycomb-structured monolayer (with a binding energy similar to that observed on the 4x4 Ag(111) surface) as well as a new form dubbed "polygonal silicene", its structure consisting of 3-, 4-, 5- and 6-sided polygons.
The p-d hybridisation mechanism between Ag and Si is important to stabilise the nearly flat silicon clusters and the effectiveness of Ag substrate for silicene growth explained by DFT calculations and molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the Motion (physics), physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamics ( ...
simulations. The unique hybridized electronic structures of epitaxial 4 × 4 silicene on Ag(111) determines highly chemical reactivity of silicene surface, which are revealed by scanning tunneling microscopy and angle-resolved photoemission spectroscopy. The hybridization between Si and Ag results in a metallic surface state, which can gradually decay due to oxygen adsorption. X-ray photoemission spectroscopy confirms the decoupling of Si-Ag bonds after oxygen treatment as well as the relative oxygen resistance of Ag(111) surface, in contrast to 4 × 4 silicene ith respect to Ag(111)
Functionalized silicene
Beyond the pure silicene structure, research into functionalized silicene has yielded successful growth of organomodified silicene – oxygen-free silicene sheets functionalized with phenyl rings. Such functionalization allows uniform dispersion of the structure inorganic solvent
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
s and indicates the potential for a range of new functionalized silicon systems and organosilicon nanosheets.
Silicene transistors
The U.S. Army Research Laboratory has been supporting research on silicene since 2014. The stated goals for research efforts were to analyze atomic scale materials, such as silicene, for properties and functionalities beyond existing materials, like graphene. In 2015, Deji Akinwande, led researchers at the University of Texas, Austin in conjunction with Alessandro Molle's group at CNR, Italy, and collaboration with U.S. Army Research Laboratory and developed a method to stabilize silicene in air and reported a functional silicenefield effect transistor
The field-effect transistor (FET) is a type of transistor that uses an electric field to control the current through a semiconductor. It comes in two types: junction FET (JFET) and metal-oxide-semiconductor FET (MOSFET). FETs have three termi ...
device. An operational transistor's material must have bandgaps, and functions more effectively if it possesses a high mobility of electrons. A bandgap is an area between the valence and conduction bands in a material where no electrons exist. Although graphene has a high mobility of electrons, the process of forming a bandgap in the material reduces many of its other electric potentials.
Therefore, there have been investigations into using graphene analogues, such as silicene, as field effect transistors. Despite silicene's natural state also having a zero-band gap, Akinwande and Molle and coworkers in collaboration with U.S. Army Research Laboratory have developed a silicene transistor. They designed a process termed “silicene encapsulated delamination with native electrodes” (SEDNE) to overcome silicene's instability in the air. The stability that resulted has been claimed to be due to Si-Ag's p-d hybridization. They grew a layer of silicene on top of a layer of Ag via epitaxy
Epitaxy (prefix ''epi-'' means "on top of”) is a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited cry ...
and covered the two with alumina (Al2O3). The silicene, Ag, and Al2O3 were stored in a vacuum at room temperature and observed over a tracked period of two months. The sample underwent Raman spectroscopy
Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra ...
to be inspected for signs of degradation, but none were found. This complex stack was then laid on top of a SiO2 substrate with the Ag facing up. Ag was removed in a thin strip down the middle to reveal a silicene channel. The silicene channel on the substrate had a life of two minutes when exposed to air until it lost its signature Raman spectra. A bandgap of approximately 210 meV was reported. The substrate's effects on silicene, in developing the bandgap, have been explained by the scattering of grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional crystallographic defect, defects in the crystal structure, and tend to decrease the ...
and limited transport of acoustic phonon
A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. In the context of optically trapped objects, the quantized vibration mode can be defined a ...
s, as well as by symmetry breaking and hybridization effect between silicene and the substrate. Acoustic phonons describe the synchronous movement of two or more types of atoms from their equilibrium position in a lattice structure.
Silicene nanosheets
2D silicene nanosheets are used in high-voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
symmetric supercapacitors as attractive electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
materials.
See also
* 2D silica * Borophene * Germanene * Stanene * PlumbeneReferences
External links
* * {{Authority control Allotropes of silicon Two-dimensional nanomaterials Group IV semiconductors Substances discovered in the 2010s