Robinson Annulation on:
[Wikipedia]
[Google]
[Amazon]
The Robinson annulation is a  Formation of cyclohexenone and derivatives are important in
Formation of cyclohexenone and derivatives are important in
 The original procedure of the Robinson annulation begins with the
The original procedure of the Robinson annulation begins with the
 It has been postulated that the difference in the formation of these transition states and their corresponding products is due to solvent interactions. Scanio found that changing the solvent of the reaction from dioxane to DMSO gives different stereochemistry in step D above. This suggests that the presence of protic or aprotic solvents gives rise to different transition states.
It has been postulated that the difference in the formation of these transition states and their corresponding products is due to solvent interactions. Scanio found that changing the solvent of the reaction from dioxane to DMSO gives different stereochemistry in step D above. This suggests that the presence of protic or aprotic solvents gives rise to different transition states.
 Robinson annulation is one notable example of a wider class of chemical transformations termed Tandem Michael-aldol reactions, that sequentially combine Michael addition and aldol reaction into a single reaction. As is the case with Robinson annulation, Michael addition usually happens first to tether the two reactants together, then aldol reaction proceeds intramolecularly to generate the ring system in the product. Usually five- or six-membered rings are generated.
Robinson annulation is one notable example of a wider class of chemical transformations termed Tandem Michael-aldol reactions, that sequentially combine Michael addition and aldol reaction into a single reaction. As is the case with Robinson annulation, Michael addition usually happens first to tether the two reactants together, then aldol reaction proceeds intramolecularly to generate the ring system in the product. Usually five- or six-membered rings are generated.




 Wang, et al. reported the one-pot synthesis of chiral thiochromenes by such an organocatalytic Robinson annulation.
Wang, et al. reported the one-pot synthesis of chiral thiochromenes by such an organocatalytic Robinson annulation.
 Scientists at Merck discovered platensimycin, a novel antibiotic lead compound with potential medicinal applications as seen in the adjacent picture.
Initial synthesis gave a racemic form of the compound using an intramolecular etherification reaction of the alcohol motifs and the double bond. Yamamoto and coworkers report the use of an alternative intramolecular Robinson annulation to provide a straightforward enantioselective synthesis of tetracyclic core of platensimycin. The key Robinson annulation step was reported to be accomplished in one pot using L-proline for chiral control. The reaction conditions can be seen below.
Scientists at Merck discovered platensimycin, a novel antibiotic lead compound with potential medicinal applications as seen in the adjacent picture.
Initial synthesis gave a racemic form of the compound using an intramolecular etherification reaction of the alcohol motifs and the double bond. Yamamoto and coworkers report the use of an alternative intramolecular Robinson annulation to provide a straightforward enantioselective synthesis of tetracyclic core of platensimycin. The key Robinson annulation step was reported to be accomplished in one pot using L-proline for chiral control. The reaction conditions can be seen below.

chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
used in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
for their application to the synthesis of many natural products and other interesting organic compounds such as antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
and steroids
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes that alter mem ...
. Specifically, the synthesis of cortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase ...
is completed through the use of the Robinson annulation.
The initial paper on the Robinson annulation was published by William Rapson and Robert Robinson while Rapson studied at Oxford with professor Robinson. Before their work, cyclohexenone syntheses were not derived from the α,β-unsaturated ketone component. Initial approaches coupled the methyl vinyl ketone with a naphthol to give a naphtholoxide, but this procedure was not sufficient to form the desired cyclohexenone. This was attributed to unsuitable conditions of the reaction.
Robinson and Rapson found in 1935 that the interaction between cyclohexanone and α,β-unsaturated ketone afforded the desired cyclohexenone. It remains one of the key methods for the construction of six membered ring compounds. Since it is so widely used, there are many aspects of the reaction that have been investigated such as variations of the substrates and reaction conditions as discussed in the scope and variations section. Robert Robinson won the Nobel Prize for Chemistry in 1947 for his contribution to the study of alkaloids.
Reaction mechanism
 The original procedure of the Robinson annulation begins with the
The original procedure of the Robinson annulation begins with the nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
of a ketone in a Michael reaction on a vinyl ketone to produce the intermediate Michael adduct. Subsequent aldol type ring closure leads to the keto alcohol, which is then followed by dehydration to produce the annulation product.
In the Michael reaction, the ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
is deprotonated by a base to form an enolate nucleophile which attacks the electron acceptor (in red). This acceptor is generally an α,β-unsaturated ketone, although aldehydes, acid derivatives and similar compounds can work as well (see scope). In the example shown here, regioselectivity is dictated by the formation of the thermodynamic enolate. Alternatively, the regioselectivity is often controlled by using a β-diketone or β-ketoester as the enolate component, since deprotonation at the carbon flanked by the carbonyl groups is strongly favored. The intramolecular aldol condensation then takes place in such a way that installs the six-membered ring. In the final product, the three carbon atoms of the α,β-unsaturated system and the carbon α to its carbonyl group make up the four-carbon bridge of the newly installed ring.
In order to avoid a reaction between the original enolate and the cyclohexenone product, the initial Michael adduct is often isolated first and then cyclized to give the desired octalone in a separate step.
Stereochemistry
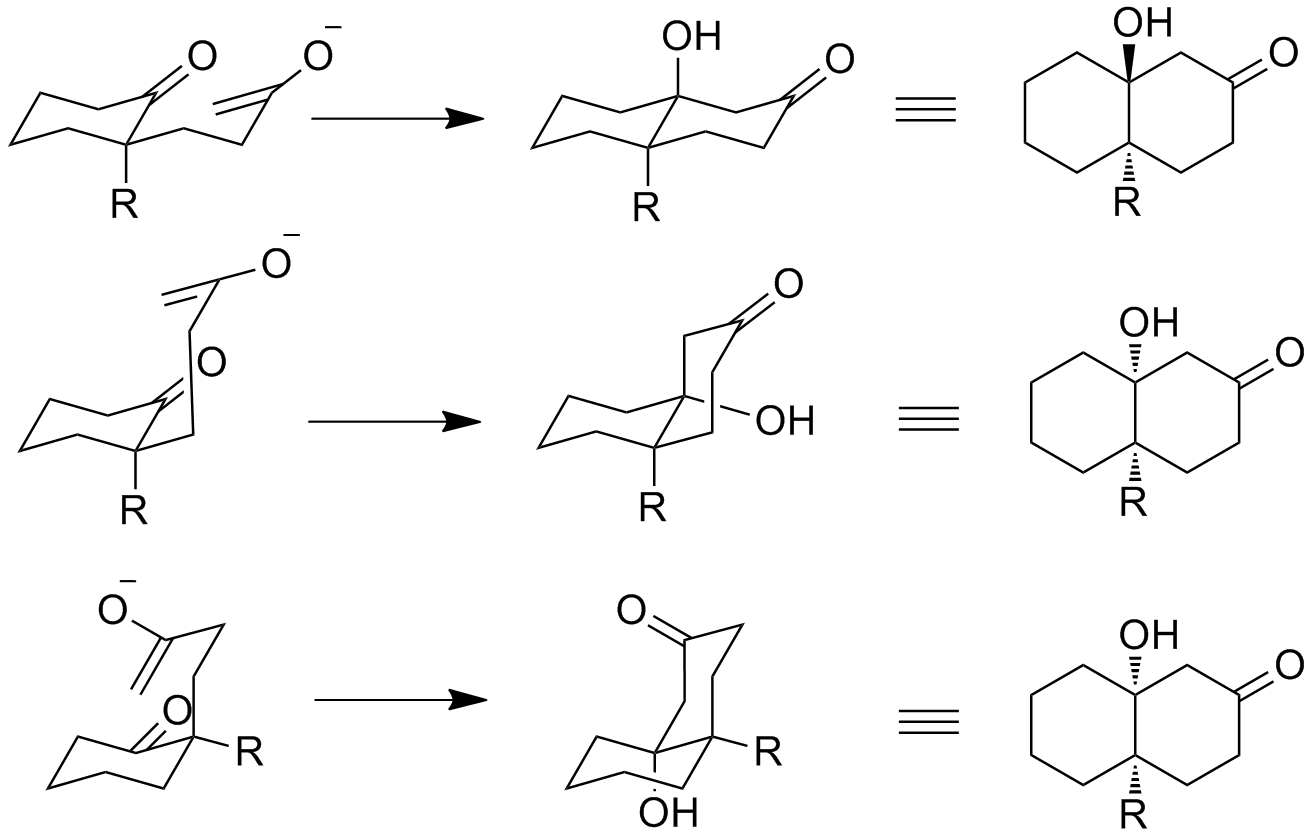
Studies have been completed on the formation of the hydroxy ketones in the Robinson annulation reaction scheme. The trans compound is favored due to antiperiplanar effects of the final aldol condensation in kinetically controlled reactions. It has also been found though that the cyclization can proceed in synclinal orientation. The figure below shows the three possible stereochemical pathways, assuming a chair transition state. It has been postulated that the difference in the formation of these transition states and their corresponding products is due to solvent interactions. Scanio found that changing the solvent of the reaction from dioxane to DMSO gives different stereochemistry in step D above. This suggests that the presence of protic or aprotic solvents gives rise to different transition states.
It has been postulated that the difference in the formation of these transition states and their corresponding products is due to solvent interactions. Scanio found that changing the solvent of the reaction from dioxane to DMSO gives different stereochemistry in step D above. This suggests that the presence of protic or aprotic solvents gives rise to different transition states.
Mechanistic classification
 Robinson annulation is one notable example of a wider class of chemical transformations termed Tandem Michael-aldol reactions, that sequentially combine Michael addition and aldol reaction into a single reaction. As is the case with Robinson annulation, Michael addition usually happens first to tether the two reactants together, then aldol reaction proceeds intramolecularly to generate the ring system in the product. Usually five- or six-membered rings are generated.
Robinson annulation is one notable example of a wider class of chemical transformations termed Tandem Michael-aldol reactions, that sequentially combine Michael addition and aldol reaction into a single reaction. As is the case with Robinson annulation, Michael addition usually happens first to tether the two reactants together, then aldol reaction proceeds intramolecularly to generate the ring system in the product. Usually five- or six-membered rings are generated.
Scope and variations
Reaction conditions
Although the Robinson annulation is generally conducted under basic conditions, reactions have been conducted under a variety of conditions. Heathcock and Ellis report similar results to the base-catalyzed method usingsulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
. The Michael reaction can occur under neutral conditions through an enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
. A Mannich base can be heated in the presence of the ketone to produce the Michael adduct. Successful preparation of compounds using the Robinson annulation methods have been reported.
The Michael acceptor
A typical Michael acceptor is an α,β-unsaturated ketone, although aldehydes and acid derivatives work as well. In addition, Bergmann ''et al.'' reports that donors such asnitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called "propionitrile" (or pro ...
, nitro compounds, sulfones and certain hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s can be used as acceptors. Overall, Michael acceptors are generally activated olefins such as those shown below where EWG refers to an electron withdrawing group such as cyano, keto, or ester as shown.
Wichterle reaction
The Wichterle reaction is a variant of the Robinson annulation that replaces methyl vinyl ketone with 1,3-dichloro-''cis''-2-butene. This gives an example of using a different Michael acceptor from the typical α,β-unsaturated ketone. The 1,3-dichloro-''cis''-2-butene is employed to avoid undesirable polymerization or condensation during the Michael addition.Hauser annulation
The reaction sequence in the related Hauser annulation is a Michael addition followed by a Dieckmann condensation and finally an elimination. The Dieckmann condensation is a similar ring closing intramolecular chemical reaction of diesters with base to give β-ketoesters. The Hauser donor is an aromatic sulfone or methylene sulfoxide with a carboxylic ester group in the ortho position. The Hauser acceptor is a Michael acceptor. In the original Hauser publication ethyl 2-carboxybenzyl phenyl sulfoxide reacts with pent-3-ene-2-one with LDA as a base in THF at −78 °C.Asymmetric Robinson annulation
Asymmetric synthesis of Robinson annulation products most often involve the use of a prolinecatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. Studies report the use of L-proline as well as several other chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
amines for use as catalysts during both steps of the Robinson annulation reaction. The advantages of using the optically active proline catalysis is that they are stereoselective with enantiomeric excesses of 60–70%.

 Wang, et al. reported the one-pot synthesis of chiral thiochromenes by such an organocatalytic Robinson annulation.
Wang, et al. reported the one-pot synthesis of chiral thiochromenes by such an organocatalytic Robinson annulation.
Applications to synthesis
The Wieland–Miescher ketone is the Robinson annulation product of 2-methyl-cyclohexane-1,3-dione and methyl vinyl ketone. This compound is used in the syntheses of manysteroids
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes that alter mem ...
possessing important biological properties and can be made enantiopure using proline catalysis.
F. Dean Toste and co-workers have used Robinson annulation in the total synthesis of (+)-fawcettimine, a tetracyclic Lycopodium alkaloid that has potential application to inhibiting the acetylcholine esterase.
Enantioselective route to platensimycin

References
{{reflist Name reactions Addition reactions Carbon-carbon bond forming reactions