remineralization on:
[Wikipedia]
[Google]
[Amazon]
In
+ Oxidant -> + \underset + \underset
The above generic equation starts with two reactants: some piece of organic matter (composed of organic carbon) and an oxidant. Most organic carbon exists in a reduced form which is then oxidized by the oxidant (such as ) into and energy that can be harnessed by the organism. This process generally produces , water and a collection of simple nutrients like nitrate or phosphate that can then be taken up by other organisms. The above general form, when considering as the oxidant, is the equation for respiration. In this context specifically, the above equation represents bacterial respiration though the reactants and products are essentially analogous to the short-hand equations used for multi-cellular respiration.
 A quarter of all organic material that exits the
A quarter of all organic material that exits the
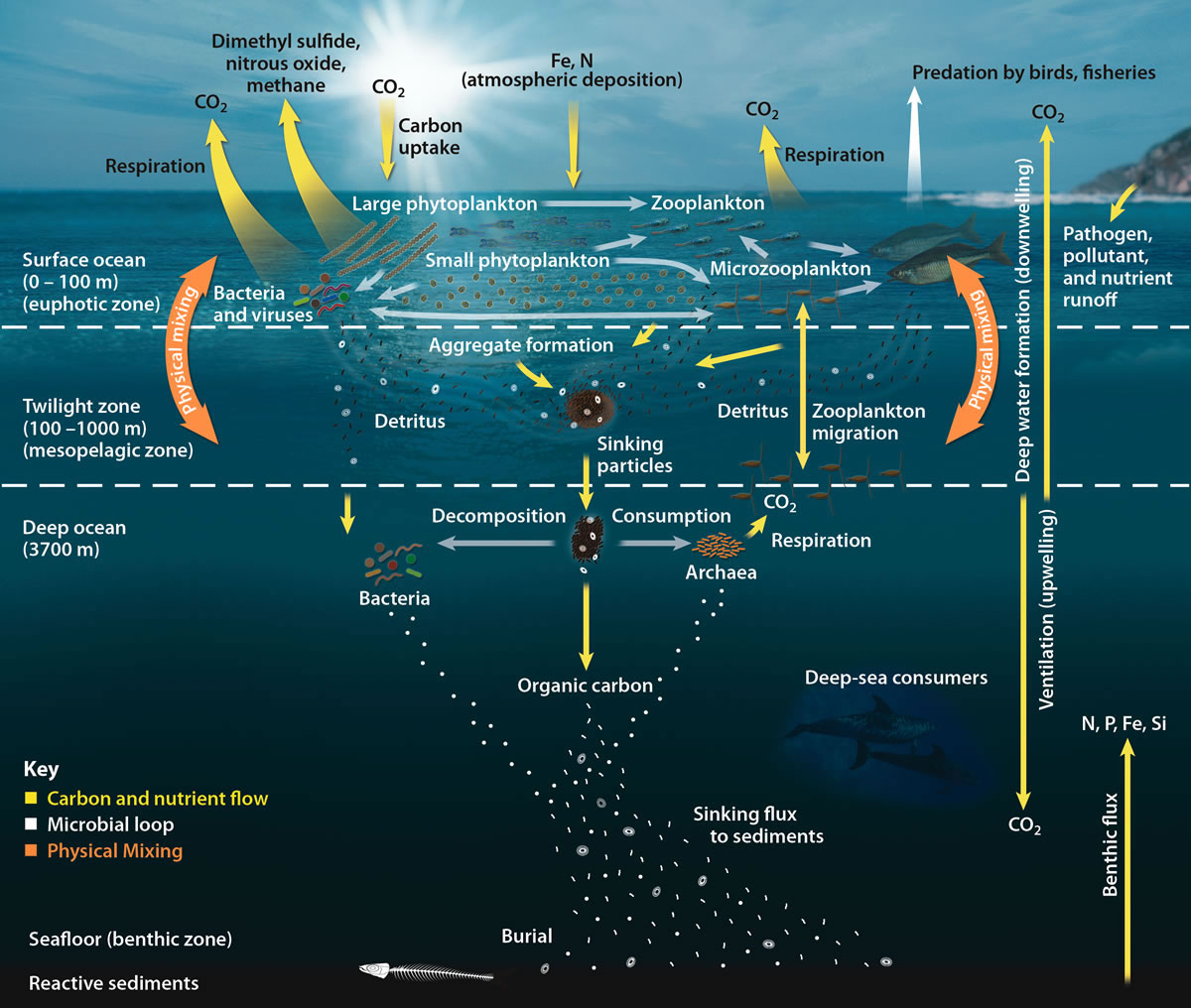 In most open ocean ecosystems only a small fraction of organic matter reaches the seafloor. Biological activity in the photic zone of most water bodies tends to recycle material so well that only a small fraction of organic matter ever sinks out of that top photosynthetic layer. Remineralisation within this top layer occurs rapidly and due to the higher concentrations of organisms and the availability of light, those remineralised nutrients are often taken up by autotrophs just as rapidly as they are released.
What fraction does escape varies depending on the location of interest. For example, in the North Sea, values of carbon deposition are ~1% of primary production while that value is <0.5% in the open oceans on average. Therefore, most of nutrients remain in the water column, recycled by the biota.
In most open ocean ecosystems only a small fraction of organic matter reaches the seafloor. Biological activity in the photic zone of most water bodies tends to recycle material so well that only a small fraction of organic matter ever sinks out of that top photosynthetic layer. Remineralisation within this top layer occurs rapidly and due to the higher concentrations of organisms and the availability of light, those remineralised nutrients are often taken up by autotrophs just as rapidly as they are released.
What fraction does escape varies depending on the location of interest. For example, in the North Sea, values of carbon deposition are ~1% of primary production while that value is <0.5% in the open oceans on average. Therefore, most of nutrients remain in the water column, recycled by the biota.
biogeochemistry
Biogeochemistry is the Branches of science, scientific discipline that involves the study of the chemistry, chemical, physics, physical, geology, geological, and biology, biological processes and reactions that govern the composition of the natu ...
, remineralisation (or remineralization) refers to the breakdown or transformation of organic matter
Organic matter, organic material or natural organic matter is the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come fro ...
(those molecules derived from a biological source) into its simplest inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
forms. These transformations form a crucial link within ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and en ...
s as they are responsible for liberating the energy stored in organic molecules and recycling matter within the system to be reused as nutrient
A nutrient is a substance used by an organism to survive, grow and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi and protists. Nutrients can be incorporated into cells for metabolic purposes or excret ...
s by other organism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s.
Remineralisation is normally viewed as it relates to the cycling of the major biologically important elements such as carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
. While crucial to all ecosystems, the process receives special consideration in aquatic settings, where it forms a significant link in the biogeochemical dynamics and cycling of aquatic ecosystems.
Role in biogeochemistry
The term "remineralization" is used in several contexts across different disciplines. The term is most commonly used in themedicinal
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
and physiological
Physiology (; ) is the science, scientific study of function (biology), functions and mechanism (biology), mechanisms in a life, living system. As a branches of science, subdiscipline of biology, physiology focuses on how organisms, organ syst ...
fields, where it describes the development or redevelopment of mineralized structures in organisms such as teeth
A tooth (: teeth) is a hard, calcified structure found in the jaws (or mouths) of many vertebrates and used to break down food. Some animals, particularly carnivores and omnivores, also use teeth to help with capturing or wounding prey, tear ...
or bone. In the field of biogeochemistry
Biogeochemistry is the Branches of science, scientific discipline that involves the study of the chemistry, chemical, physics, physical, geology, geological, and biology, biological processes and reactions that govern the composition of the natu ...
, however, remineralization is used to describe a link in the chain of elemental cycling within a specific ecosystem. In particular, remineralization represents the point where organic material constructed by living organisms is broken down into basal inorganic components that are not obviously identifiable as having come from an organic source. This differs from the process of decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
which is a more general descriptor of larger structures degrading to smaller structures.
Biogeochemists study this process across all ecosystems for a variety of reasons. This is done primarily to investigate the flow of material and energy in a given system, which is key to understanding the productivity of that ecosystem along with how it recycles material versus how much is entering the system. Understanding the rates and dynamics of organic matter remineralization in a given system can help in determining how or why some ecosystems might be more productive than others.
Remineralization reactions
While it is important to note that the process of remineralization is a series of complex biochemical pathways ithin microbes it can often be simplified as a series of one-step processes for ecosystem-level models and calculations. A generic form of these reactions is shown by: :Electron acceptor cascade
The degradation of organic matter through respiration in the modern ocean is facilitated by different electron acceptors, their favorability based on Gibbs free energy law, and thelaws of thermodynamics
The laws of thermodynamics are a set of scientific laws which define a group of physical quantities, such as temperature, energy, and entropy, that characterize thermodynamic systems in thermodynamic equilibrium. The laws also use various param ...
. This redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
chemistry is the basis for life in deep sea
The deep sea is broadly defined as the ocean depth where light begins to fade, at an approximate depth of or the point of transition from continental shelves to continental slopes. Conditions within the deep sea are a combination of low tempe ...
sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
s and determines the obtainability of energy to organisms that live there. From the water interface moving toward deeper sediments, the order of these acceptors is oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
, manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, and sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
. The zonation of these favored acceptors can be seen in Figure 1. Moving downwards from the surface through the zonation of these deep ocean sediments, acceptors are used and depleted. Once depleted the next acceptor of lower favorability takes its place. Thermodynamically, oxygen represents the most favorable electron accepted but is quickly used up in the water sediment interface and concentrations extends only millimeters to centimeters down into the sediment in most locations of the deep sea. This favorability indicates an organism's ability to obtain higher energy from the reaction which helps them compete with other organisms. In the absence of these acceptors, organic matter can also be degraded through methanogenesis, but the net oxidation of this organic matter is not fully represented by this process. Each pathway and the stoichiometry of its reaction are listed in table 1.
Due to this quick depletion of in the surface sediments, a majority of microbes use anaerobic pathways to metabolize other oxides such as manganese, iron, and sulfate. It is also important to figure in bioturbation
Bioturbation is defined as the reworking of soils and sediments by animals or plants. It includes burrowing, ingestion, and defecation of sediment grains. Bioturbating activities have a profound effect on the environment and are thought to be a ...
and the constant mixing of this material which can change the relative importance of each respiration pathway. For the microbial perspective please reference the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
.
Remineralisation in sediments
Reactions
 A quarter of all organic material that exits the
A quarter of all organic material that exits the photic zone
The photic zone (or euphotic zone, epipelagic zone, or sunlight zone) is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological ...
makes it to the seafloor without being remineralised and 90% of that remaining material is remineralised in sediments itself. Once in the sediment, organic remineralisation may occur through a variety of reactions. The following reactions are the primary ways in which organic matter is remineralised, in them general organic matter (OM) is often represented by the shorthand: .
Aerobic respiration
Aerobic respiration is the most preferred remineralisation reaction due to its high energy yield. Although oxygen is quickly depleted in the sediments and is generally exhausted centimeters from the sediment-water interface.Anaerobic respiration
In instances in which the environment is suboxic oranoxic
Anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of dissolved ox ...
, organisms will prefer to utilize denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitr ...
to remineralise organic matter as it provides the second largest amount of energy. In depths below where denitrification is favored, reactions such as Manganese Reduction, Iron Reduction, Sulfate Reduction, Methane Reduction (also known as Methanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. It is the fourth and final stage of anaerobic digestion. Organisms capable of producing methane for energy conservation h ...
), become favored respectively. This favorability is governed by Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
(ΔG). In a water body, sediment seabed, or soil, the sorting of these chemical reactions with depth in order of energy provided is called a redox gradient.
Redox zonation
Redox zonation refers to how the processes that transfer terminal electrons as a result of organic matter degradation vary depending on time and space. Certain reactions will be favored over others due to their energy yield as detailed in the energy acceptor cascade detailed above. In oxic conditions, in which oxygen is readily available, aerobic respiration will be favored due to its high energy yield. Once the use of oxygen through respiration exceeds the input of oxygen due to bioturbation and diffusion, the environment will become anoxic and organic matter will be broken down via other means, such as denitrification and manganese reduction.Remineralisation in the open ocean
 In most open ocean ecosystems only a small fraction of organic matter reaches the seafloor. Biological activity in the photic zone of most water bodies tends to recycle material so well that only a small fraction of organic matter ever sinks out of that top photosynthetic layer. Remineralisation within this top layer occurs rapidly and due to the higher concentrations of organisms and the availability of light, those remineralised nutrients are often taken up by autotrophs just as rapidly as they are released.
What fraction does escape varies depending on the location of interest. For example, in the North Sea, values of carbon deposition are ~1% of primary production while that value is <0.5% in the open oceans on average. Therefore, most of nutrients remain in the water column, recycled by the biota.
In most open ocean ecosystems only a small fraction of organic matter reaches the seafloor. Biological activity in the photic zone of most water bodies tends to recycle material so well that only a small fraction of organic matter ever sinks out of that top photosynthetic layer. Remineralisation within this top layer occurs rapidly and due to the higher concentrations of organisms and the availability of light, those remineralised nutrients are often taken up by autotrophs just as rapidly as they are released.
What fraction does escape varies depending on the location of interest. For example, in the North Sea, values of carbon deposition are ~1% of primary production while that value is <0.5% in the open oceans on average. Therefore, most of nutrients remain in the water column, recycled by the biota. Heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but ...
ic organisms will utilize the materials produced by the autotroph
An autotroph is an organism that can convert Abiotic component, abiotic sources of energy into energy stored in organic compounds, which can be used by Heterotroph, other organisms. Autotrophs produce complex organic compounds (such as carbohy ...
ic (and chemotroph
A chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic ( chemoorganotrophs) or inorganic ( chemolithotrophs). The chemotroph designation is in contrast to phot ...
ic) organisms and via respiration will remineralise the compounds from the organic form back to inorganic, making them available for primary producers again.
For most areas of the ocean, the highest rates of carbon remineralisation occur at depths between in the water column, decreasing down to about 1,200 m where remineralisation rates remain pretty constant at 0.1 μmol kg−1 yr−1. As a result of this, the pool of remineralised carbon (which generally takes the form of carbon dioxide) tends to increase in the photic zone.
Most remineralisation is done with dissolved organic carbon
Dissolved organic carbon (DOC) is the fraction of organic carbon Operational definition, operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometre, micrometers. The fraction remain ...
(DOC). Studies have shown that it is larger sinking particles that transport matter down to the sea floor while suspended particles and dissolved organics are mostly consumed by remineralisation. This happens in part due to the fact that organisms must typically ingest nutrients smaller than they are, often by orders of magnitude. With the microbial community making up 90% of marine biomass, it is particles smaller than the microbes (on the order of ) that will be taken up for remineralisation.
See also
*Biological pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven Carbon sequestration, sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sedim ...
* Decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
* f-ratio F-ratio or f-ratio may refer to:
* The F-ratio used in statistics, which relates the variances of independent samples; see F-distribution
* f-ratio (oceanography), which relates recycled and total primary production in the surface ocean
* f-number ...
* John D. Hamaker (soil remineralisation)
* Mineralization (biology)
Biomineralization, also written biomineralisation, is the process by which living organisms produce minerals, often resulting in hardened or stiffened '' mineralized tissues''. It is an extremely widespread phenomenon: all six taxonomic kingd ...
* Mineralization (soil science)
* Immobilization (soil science)
Immobilization in soil science is the conversion of inorganic compounds to organic compounds by microorganisms or plants by which the compounds become inaccessible to plants. Immobilization is the opposite of mineralization. In immobilization, in ...
References
{{reflist Biogeochemistry Oceanography Limnology