|
Denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitrification as a type of respiration that reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O) finally resulting in the production of dinitrogen (N2) completing the nitrogen cycle. Denitrifying microbes require a very low oxygen concentration of less than 10%, as well as organic C for energy. Since denitrification can remove NO3−, reducing its leaching to groundwater, it can be strategically used to treat sewage or animal residues of high nitrogen content. Denitrification can leak N2O, which is an ozone-d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dissimilatory Nitrate Reduction To Ammonium
Dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification, is the result of anaerobic respiration by chemoorganoheterotrophic microbes using nitrate (NO3−) as an electron acceptor for respiration. In anaerobic conditions microbes which undertake DNRA oxidise organic matter and use nitrate (rather than oxygen) as an electron acceptor, reducing it to nitrite, and then to ammonium (NO3− → NO2− → NH4+). Dissimilatory nitrate reduction to ammonium is more common in prokaryotes but may also occur in eukaryotic microorganisms. DNRA is a component of the terrestrial and oceanic nitrogen cycle. Unlike denitrification, it acts to conserve bioavailable nitrogen in the system, producing soluble ammonium rather than unreactive nitrogen gas (). Background and process Cellular process Dissimilatory nitrate reduction to ammonium is a two step process, reducing NO3− to NO2− then NO2− to NH4+, though the reaction may begin with NO2− direc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen Cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include nitrogen fixation, fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Paracoccus Denitrificans
''Paracoccus denitrificans'', is a coccoid bacterium known for its nitrate reducing properties, its ability to replicate under conditions of hypergravity and for being a relative of the eukaryotic mitochondrion (endosymbiotic theory). Description ''Paracoccus denitrificans'', is a gram-negative, coccus, non-motile, denitrifying (nitrate-reducing) bacterium. It is typically a rod-shaped bacterium but assumes spherical shapes during the stationary phase. Like all gram-negative bacteria, it has a double membrane with a cell wall. Formerly known as ''Micrococcus denitrificans'', it was first isolated in 1910 by Martinus Beijerinck, a Dutch microbiologist. The bacterium was reclassified in 1969 to ''Paracoccus denitrificans'' by D.H. Davis. The genome of ''P. denitrificans'' was sequenced in 2004. Ecology and ecological applications Metabolically ''Paracoccus denitrificans'' is very flexible and has been recorded in soil in both aerobic or anaerobic environments. The microbe also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen Cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include nitrogen fixation, fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrous-oxide Reductase
In enzymology, a nitrous oxide reductase also known as nitrogen:acceptor oxidoreductase (N2O-forming) is an enzyme that catalyzes the final step in bacterial denitrification, the reduction of nitrous oxide to dinitrogen. : N2O + 2 reduced cytochome ''c'' N2 + H2O + 2 cytochrome ''c'' It plays a critical role in preventing release of a potent greenhouse gas into the atmosphere. Function N2O is an inorganic metabolite of the prokaryotic cell during denitrification. Thus, denitrifiers comprise the principal group of N2O producers, with roles played also by nitrifiers, methanotrophic bacteria, and fungi. Among them, only denitrifying prokaryotes have the ability to convert N2O to N2. Conversion of N2O into N2 is the last step of a complete nitrate denitrification process and is an autonomous form of respiration. N2O is generated in the denitrifying cell by the activity of respiratory NO reductase. Some microbial communities only have the capability of N2O reduction to N2 and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room temperature, it is a colourless Flammability#Definitions, non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful Oxidising agent, oxidiser similar to molecular oxygen. Nitrous oxide has significant Nitrous oxide (medication), medical uses, especially in surgery and dentistry, for its Anesthesia, anaesthetic and Analgesic, pain-reducing effects, and it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Its colloquial name, "laughing gas", coined by Humphry Davy, describes the Euphoria, euphoric effects upon inhaling it, which cause it to be used as a recreational drug inducing a brief "Dissociative, high". When abused chronically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of Resonance (chemistry), resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dinitrogen
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colourless and odourless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitric-oxide Reductase
Nitric oxide reductase, an enzyme, catalyzes the reduction of nitric oxide (NO) to nitrous oxide (N2O). The enzyme participates in nitrogen metabolism and in the microbial defense against nitric oxide toxicity. The catalyzed reaction may be dependent on different participating small molecules: Cytochrome c (EC: 1.7.2.5, Nitric oxide reductase (cytochrome c)), NADPH (EC:1.7.1.14), or Menaquinone (EC:1.7.5.2). Nomenclature Nitric oxide reductase was assigned Enzyme Commission number (EC) 1.7.2.5. Enzyme Commission numbers are the standard naming system used for enzymes. The EC identifies the class, subclass, sub-subclass, and serial number of the enzyme. Nitric oxide reductase is in Class 1, therefore it is an oxidoreductases. Nitric oxide reductase belongs to the family of oxidoreductases, specifically those acting on other nitrogenous compounds as donors with other acceptors. The systematic name of this enzyme class is nitrous-oxide:acceptor oxidoreductase (NO-forming) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
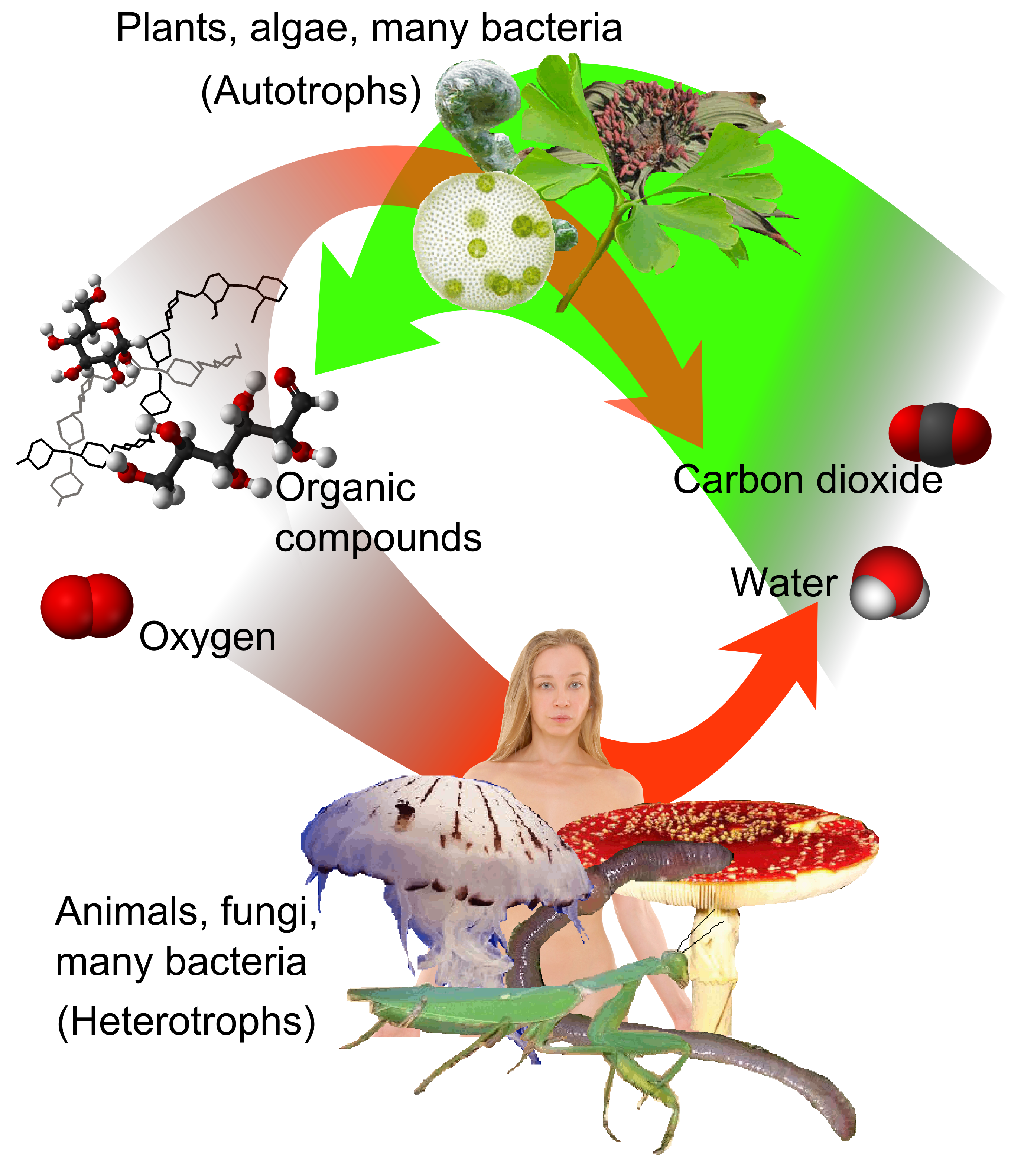
Heterotrophic
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but not producers. Living organisms that are heterotrophic include all animals and fungi, some bacteria and protists, and many parasitic plants. The term heterotroph arose in microbiology in 1946 as part of a classification of microorganisms based on their type of nutrition. The term is now used in many fields, such as ecology, in describing the food chain. Heterotrophs occupy the second and third trophic levels of the food chain while autotrophs occupy the first trophic level. Heterotrophs may be subdivided according to their energy source. If the heterotroph uses chemical energy, it is a chemoheterotroph (e.g., humans and mushrooms). If it uses light for energy, then it is a photoheterotroph (e.g., green non-sulfur bacteria). Heterotrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and energy flows. Ecosystems are controlled by external and internal Environmental factor, factors. External factors—including climate—control the ecosystem's structure, but are not influenced by it. By contrast, internal factors control and are controlled by ecosystem processes; these include decomposition, the types of species present, root competition, shading, disturbance, and succession. While external factors generally determine which Resource (biology), resource inputs an ecosystem has, their availability within the ecosystem is controlled by internal factors. Ecosystems are wikt:dynamic, dynamic, subject to periodic disturbances and always in the process of recovering from past disturbances. The tendency of an ecosystem to remain clo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |