Porphyrin Complexes on:
[Wikipedia]
[Google]
[Amazon]
220px, A picket-fence porphyrin complex of Fe, with axial coordination sites occupied by methylimidazole (green) and dioxygen (R = amide groups).
Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of
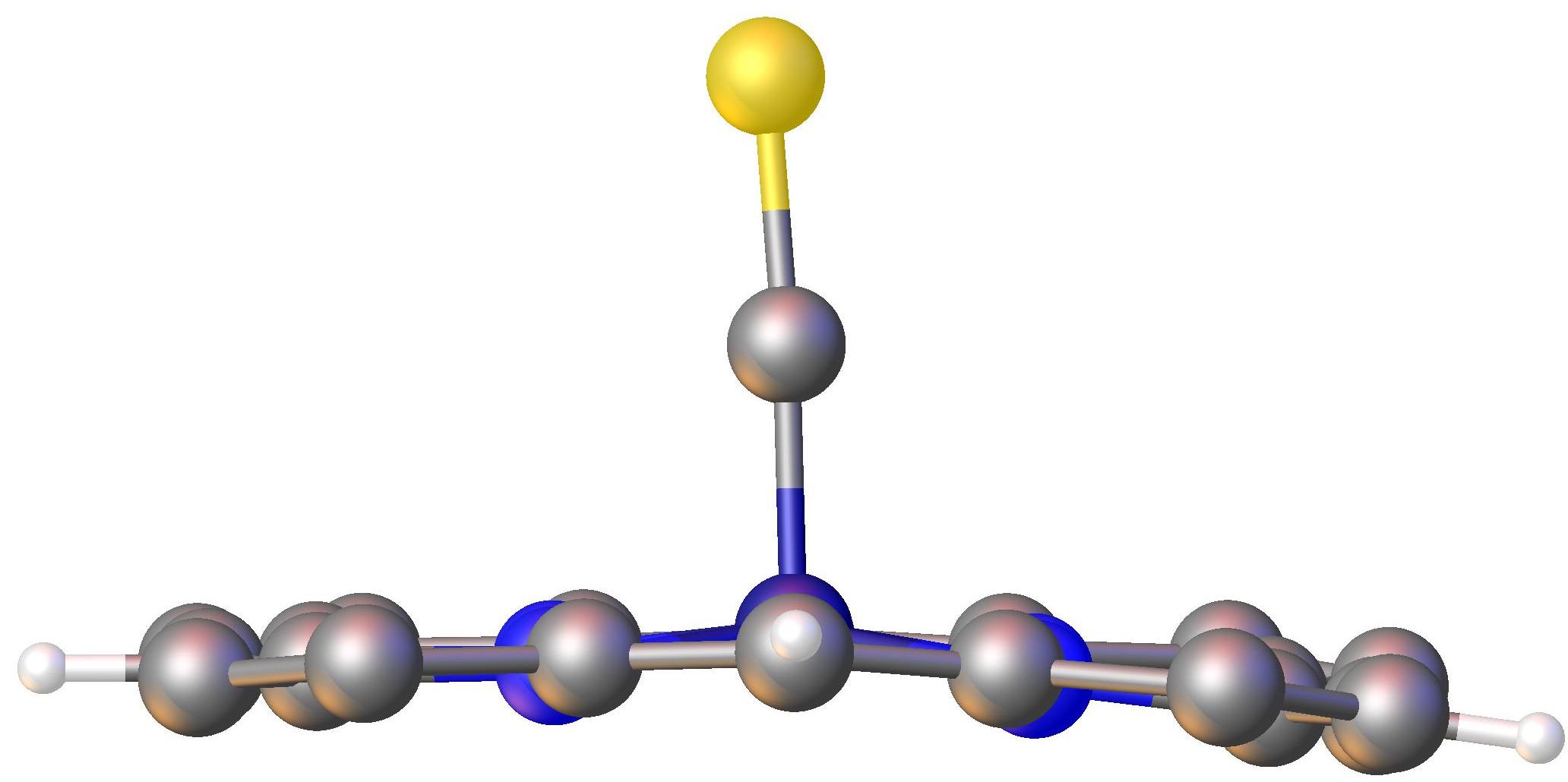 Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display only small deviations from planarity. Additionally, the metal is often not centered in the N4 plane.
Large metals such as zirconium, tantalum, and molybdenum tend to bind ''two'' porphyrin ligands. Some (OEP)sub>2 feature a multiple bonds between the metals.
Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display only small deviations from planarity. Additionally, the metal is often not centered in the N4 plane.
Large metals such as zirconium, tantalum, and molybdenum tend to bind ''two'' porphyrin ligands. Some (OEP)sub>2 feature a multiple bonds between the metals.
porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, w ...
s. Iron porphyrin complexes occur widely in nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest.
Structure
 Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display only small deviations from planarity. Additionally, the metal is often not centered in the N4 plane.
Large metals such as zirconium, tantalum, and molybdenum tend to bind ''two'' porphyrin ligands. Some (OEP)sub>2 feature a multiple bonds between the metals.
Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display only small deviations from planarity. Additionally, the metal is often not centered in the N4 plane.
Large metals such as zirconium, tantalum, and molybdenum tend to bind ''two'' porphyrin ligands. Some (OEP)sub>2 feature a multiple bonds between the metals.
Formation
Metal porphyrin complexes are almost always prepared by direct reaction of a metal halide with the free porphyrin, abbreviated here as H2P: :MClx + H2P → M(P)Cl2−x + 2HCl Two pyrrole protons are lost. The porphyrin dianion is an L2X2 ligand. These syntheses require somewhat forcing conditions, consistent with the tight fit of the metal in the N42- "pocket." In nature, the insertion is mediated bychelatase
In biochemistry, chelatases are enzymes that catalyze the insertion ("metalation") of naturally occurring tetrapyrroles. Many tetrapyrrole-based cofactors exist in nature including hemes, chlorophylls, and vitamin B12. These metallo cofactors are ...
enzymes. The insertion of a metal in synthetic porphyrins proceeds by the intermediacy of a "sitting atop complex" (SAC), whereby the entering metal interacts with only one or a two of the pyrrolic nitrogen centers.
In contrast to natural porphyrins, synthetic porphyrin ligands are typically symmetrical (i.e., their dianionic conjugate bases). Two major varieties are well studied, those with substituents at the meso positions, the premier example being tetraphenylporphyrin
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin ...
. These ligands are easy to prepare in one-pot procedures. A large number of aryl groups can be deployed aside from phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
.
A second class of synthetic porphyrins have hydrogen at the meso positions. Octaethylporphyrin
Octaethylporphyrin (H2OEP) is an organic compound that is a relative of naturally occurring heme pigments. The compound is used in the preparation of models for the prosthetic group in heme proteins. It is a dark purple solid that is soluble in ...
(H2OEP) is the subject of many such studies. It is more expensive than tetraphenylporphyrin.
Protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not sol ...
, which occurs naturally, can be modified by removal of the vinyl groups and esterification of the carboxylic acid groups to gives deuteroporphyin IX dimethyl ester.
Biomimetic complexes
file:PPIXtransH.png, left, 192px,Protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not sol ...
is the precursor to heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
and closely related to chlorophyll.
Iron porphyrin complexes ("hemes") are the dominant metalloporphyrin complexes in nature. Consequently, synthetic iron porphyrin complexes are well investigated. Common derivatives are those of Fe(III) and Fe(II). Complexes of the type Fe(P)Cl are square-pyramidal and high spin with idealized C4v symmetry. Base hydrolysis affords the "mu-oxo dimers" with the formula e(P)sub>2O. These complexes have been widely investigated as oxidation catalysts. Typical stoichiometries of ferrous porphyrins are Fe(P)L2 where L is a neutral ligand such as pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
and imidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
Many natural products, ...
. Cobalt(II) porphyrins behave similarly to the ferrous derivatives. They bind O2 to form dioxygen complex Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes wit ...
es.
Synthetic applications
Catalysts based on synthetic metalloporphyrins have been extensively investigated, although few or no applications exist. Due to their distinctive redox properties, Co(II)–porphyrin-based systems are radical initiators. Some complexes emulate the action of variousheme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
enzymes such as cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for examp ...
, lignin peroxidase. Metalloporphyrins are also studied as catalysts for water splitting, with the purpose of generating molecular hydrogen and oxygen for fuel cells.
In addition, porous organic polymers based on porphyrins, along with metal oxide nanoparticles,
Supramolecular chemistry
Porphyrins are often used to construct structures insupramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
See also
*phthalocyanines
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity.
It is composed of four isoindole units linked by a ring of nitrogen atoms ...
* macrocyclic ligand
References