|
Porphyrin Complexes
220px, A picket-fence porphyrin complex of Fe, with axial coordination sites occupied by methylimidazole (green) and dioxygen (R = amide groups). Transition metal porphyrin complexes are a family of coordination complexes of the conjugate base of porphyrins. Iron porphyrin complexes occur widely in nature, which has stimulated extensive studies on related synthetic complexes. The metal-porphyrin interaction is a strong one such that metalloporphyrins are thermally robust. They are catalysts and exhibit rich optical properties, although these complexes remain mainly of academic interest. Structure Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display only small deviations from planarity. Additionally, the metal is often not centered in the N4 plane. Large metals such as zirconium, tantalum, and molybdenum tend to bind ''two'' porphyrin ligands. Some (OEP)sub>2 feature a multiple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protoporphyrin IX
Protoporphyrin IX is an organic compound, classified as a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like heme (hemoglobin) and chlorophyll. It is a deeply colored solid that is not soluble in water. The name is often abbreviated as PPIX. Protoporphyrin IX contains a porphine core, a tetrapyrrole macrocycle with a marked aromatic character. Protoporphyrin IX is essentially planar, except for the N-H bonds that are bent out of the plane of the rings, in opposite (trans) directions. Nomenclature The general term protoporphyrin refers to porphine derivatives that have the outer hydrogen atoms in the four pyrrole rings replaced by other functional groups. The prefix proto often means 'first' in science nomenclature (such as carbon protoxide), hence Hans Fischer is thought to have coined the name protoporphyrin as the first class of porphyrins. Fischer described iron-deprived heme becoming the "proto-" porphyrin, partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecules
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids, and nucleic acids, as well as small molecules such as vitamins and hormones. A general name for this class of material is ''biological materials''. Biomolecules are an important element of living organisms. They are often endogenous, i.e. produced within the organism, but organisms usually also need exogenous biomolecules, for example certain nutrients, to survive. Biomolecules and their reactions are studied in biology and its subfields of biochemistry and molecular biology. Most biomolecules are organic compounds, and just four elements—oxygen, carbon, hydrogen, and nitrogen—make up 96% of the human body's mass. But many other elements, such as the various biometals, are also present in small amounts. The uniformity of bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocyclic Ligand
In coordination chemistry, a macrocyclic ligand is a macrocyclic ring having at least nine atoms (including all hetero atoms) and three or more donor sites that serve as ligands. Crown ethers and porphyrins are prominent examples. Macrocyclic ligands often exhibit high affinity for metal ions, the macrocyclic effect. History Porphyrins and phthalocyanines have long been recognized as potent ligands in coordination chemistry as illustrated by numerous transition metal porphyrin complexes and the commercialization of copper phthalocyanine pigments. In the 1960s the synthesis of macrocylic ligands received much attention. One early contribution involved the synthesis of the "Curtis macrocycles", in which a metal ion serves as a template for ring formation. Polyether macrocycles - or "crown" ligands - were also developed at that time. A few years later, three-dimensional analogs of crown ethers called " cryptands" were reported by Lehn and co-workers. Macrocyclic effect Ag( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalocyanines
Phthalocyanine () is a large, aromatic, macrocyclic, organic compound with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity. It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complexes derived from , the conjugate base of , are valuable in catalysis, organic solar cells, and photodynamic therapy. Properties Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents. Benzene at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are, thermally, very stable a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocyclization. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, medium sized rings or polymers tend to form. Early macrocyclizations were achieved ketonic decarboxylations for the preparation of terpenoid macrocycles. So, while Ružička was able to produce various macrocycles, the yields were low. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Reactions amenable to high dilution include Dieckmann condensation and related based-induced reactions of esters with remote halides. Some macrocyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatics, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, coordination complex, metal coordination, hydrophobic effect, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, folding (chemistry), molecular folding, molecular recognition, host–gues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Host Guest Complex Porphyrin Sanders AngewChemIntEdEngl 1995 1096
A host is a person responsible for guests at an event or for providing hospitality during it. Host may also refer to: Places * Host, Pennsylvania, a village in Berks County * Host Island, in the Wilhelm Archipelago, Antarctica People * Jim Host (born 1937), American businessman * Michel Host (1942–2021), French writer *"Host", an author abbreviation in botany for Nicolaus Thomas Host Arts, entertainment, and media Fictional entities * Hosts (''World of Darkness''), fictional characters in game ''Werewolf: The Forsaken'' *Hosts, alien invaders and overlords in the TV series ''Colony'' *Avenging Host, a group of characters in Marvel Comics' ''Earth X'' series of comic books *Rutan Host, fictional aliens from ''Doctor Who'' Film * ''Host'' (film), a 2020 horror film directed by Rob Savage Literature *''Host'', the third novel in the Rogue Mage series by Faith Hunter *''Host'', a 1993 book by Peter James * ''Hosts'' (novel), a 2001 book written by American author F. P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
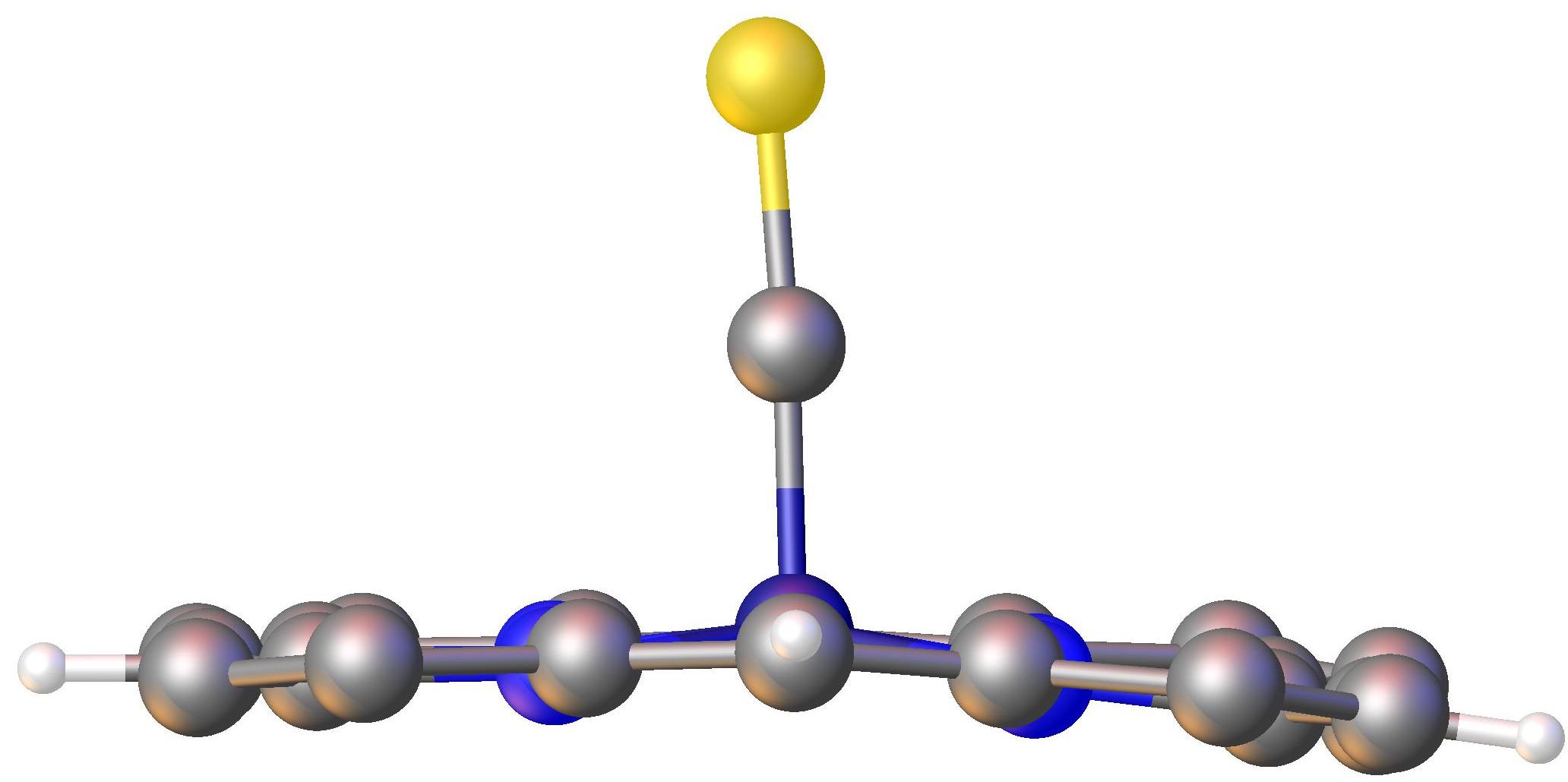
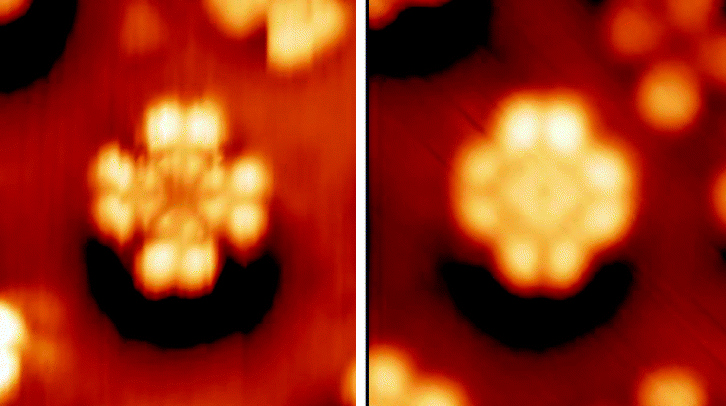
Porphyrin On Au(111) STM
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis. The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons the porphyrin ring structure is a coordinated aromatic system. One result of the large conjugated system is that porphyrins absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives . Structure Porphyrin complexes consist of a square planar MN4 core. The periph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lignin Peroxidase
In enzymology, a lignin peroxidase () is an enzyme that catalyzes the chemical reaction :1,2-bis(3,4-dimethoxyphenyl)propane-1,3-diol + H2O2 \rightleftharpoons 3,4-dimethoxybenzaldehyde + 1-(3,4-dimethoxyphenyl)ethane-1,2-diol + H2O Thus, the two substrates of this enzyme are 1,2-bis(3,4-dimethoxyphenyl)propane-1,3-diol and H2O2, whereas its 3 products are 3,4-dimethoxybenzaldehyde, 1-(3,4-dimethoxyphenyl)ethane-1,2-diol, and H2O. This enzyme belongs to the family of oxidoreductases, specifically those acting on a peroxide as acceptor (peroxidases) and can be included in the broad category of ligninases. The systematic name of this enzyme class is 1,2-bis(3,4-dimethoxyphenyl)propane-1,3-diol:hydrogen-peroxide oxidoreductase. Other names in common use include diarylpropane oxygenase, ligninase I, diarylpropane peroxidase, LiP, diarylpropane:oxygen,hydrogen-peroxide oxidoreductase (C-C-bond-cleaving). It employs one cofactor, heme. Background Lignin is highly resistant to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxygen Complex
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2. Mononuclear complexes of O2 O2 binds to a single metal center either "end-on" ( ''η''1-) or "side-on" (''η''2-). The bonding and structures of these compounds are usually evaluated by single-crystal X-ray crystallography, focusing both on the overall geometry as well as the O–O distances, which reveals the bond order of the O2 ligand. : Complexes of ''η''1-O2 ligands 220px, A picket-fence porphyrin complex of Fe, with axial coordination sites occup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |