Electrochlorination on:
[Wikipedia]
[Google]
[Amazon]
Electrochlorination is the process of producing
 Electrochlorination is the next step in the evolution of this process. It chlorinates drinking water without producing environmental
Electrochlorination is the next step in the evolution of this process. It chlorinates drinking water without producing environmental
hypochlorite
In chemistry, hypochlorite, or chloroxide is an oxyanion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite ...
by passing electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
through salt water. This disinfect
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant endospore, bacterial spores; it is less effect ...
s the water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
and makes it safe for human
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
use, such as for drinking
Drinking is the act of ingesting water or other liquids into the body through the mouth, proboscis, or elsewhere. Humans drink by swallowing, completed by peristalsis in the esophagus. The physiological processes of drinking vary widely among ...
water or swimming pool
A swimming pool, swimming bath, wading pool, paddling pool, or simply pool, is a structure designed to hold water to enable Human swimming, swimming and associated activities. Pools can be built into the ground (in-ground pools) or built abo ...
s.
Process
The process of electrochlorination is a simple application based on the chloralkali process (in an unpartitioned cell). It is theelectrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
of saltwater to produce a chlorinated solution. The first step is removing any solids from the saltwater. Next, the saltwater streams through an electrolyzer cell's channel of decreasing thickness. One side of the channel is a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
, the other is an anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
. A low voltage DC current is applied, electrolysis happens producing sodium hypochlorite
Sodium hypochlorite is an alkaline inorganic chemical compound with the formula (also written as NaClO). It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of ...
and hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
gas (H2). The solution travels to a tank
A tank is an armoured fighting vehicle intended as a primary offensive weapon in front-line ground combat. Tank designs are a balance of heavy firepower, strong armour, and battlefield mobility provided by tracks and a powerful engine; ...
that separates the hydrogen gas based on its low density. Only water and sodium chloride are used. The simplified chemical reaction is:
:NaCl + H2O + energy → NaOCl + H2
That is, energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
is added to sodium chloride
Sodium chloride , commonly known as Salt#Edible salt, edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is transparent or translucent, brittle, hygroscopic, and occurs a ...
(table salt) in water, producing sodium hypochlorite and hydrogen gas.
Because the reaction takes place in an unpartitioned cell and NaOH is present in the same solution as the Cl2:
:2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2
any Cl2 disproportionates to hypochlorite and chloride
:Cl2 + 2 NaOH → NaCl + NaClO + H2O
resulting in a hypochlorite solution.
Seawater
Companies may useseawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
for this process due to its low cost. The water used is usually brackish water or brine
Brine (or briny water) is a high-concentration solution of salt (typically sodium chloride or calcium chloride) in water. In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawat ...
(i.e. a solution with >0.5% salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensio ...
). In these cases, additional contaminant chemical
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combin ...
s may be present in the water feed. The low voltage DC current still performs electrochlorination. The excess chemicals are left untouched and can be easily discarded.
Products
The product of the process, sodium hypochlorite, provides 0.7% to 1%chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
. Anything below the concentration of 1% chlorine is considered a non-hazardous chemical although still a very effective disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than ...
. The sodium hypochlorite produced is in the range of pH 6-7.5, relatively neutral in regards to acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ity or baseness. At that pH range, the sodium hypochlorite is relatively stable.
Applications
Drinking water
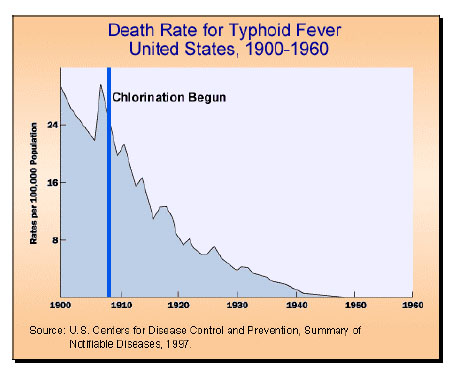
Water treatment plants have evolved their technology over the years to tackle health threats due to water contamination egcholera
Cholera () is an infection of the small intestine by some Strain (biology), strains of the Bacteria, bacterium ''Vibrio cholerae''. Symptoms may range from none, to mild, to severe. The classic symptom is large amounts of watery diarrhea last ...
, typhoid
Typhoid fever, also known simply as typhoid, is a disease caused by ''Salmonella enterica'' serotype Typhi bacteria, also called ''Salmonella'' Typhi. Symptoms vary from mild to severe, and usually begin six to 30 days after exposure. Often ther ...
, and dysentery
Dysentery ( , ), historically known as the bloody flux, is a type of gastroenteritis that results in bloody diarrhea. Other symptoms may include fever, abdominal pain, and a feeling of incomplete defecation. Complications may include dehyd ...
. Treatment plants began to implement chlorination. Chlorination virtually wiped out both the spread and initial contamination of these diseases, and did so in a way that earned it the title of "probably the most significant public health advance of the millennium" from Life Magazine.
 Electrochlorination is the next step in the evolution of this process. It chlorinates drinking water without producing environmental
Electrochlorination is the next step in the evolution of this process. It chlorinates drinking water without producing environmental toxin
A toxin is a naturally occurring poison produced by metabolic activities of living cells or organisms. They occur especially as proteins, often conjugated. The term was first used by organic chemist Ludwig Brieger (1849–1919), derived ...
s. Unlike other chlorination techniques, electrochlorination generates no sludge
Sludge (possibly , or some dialect related to slush) is a semi-solid slurry that can be produced from a range of industrial processes, from water treatment, wastewater treatment or on-site sanitation systems. It can be produced as a settled sus ...
or by-products other than hydrogen which must be managed safely. It is safer for the operators of the chlorinators as there is no handling of chlorine gas
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, which is highly toxic and corrosive. A risk assessment
Risk assessment is a process for identifying hazards, potential (future) events which may negatively impact on individuals, assets, and/or the environment because of those hazards, their likelihood and consequences, and actions which can mitigate ...
is required as the hydrogen released is flammable and explosive.
Swimming pools
When a swimmer enters a pool, they add up to one billionorganism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s to the water. Chlorination kills all organisms harmful to swimmers such as those that cause ear infection
Otitis is a general term for inflammation in ear or ear infection, inner ear infection, middle ear infection of the ear, in both humans and other animals. When infection is present, it may be viral or bacterial. When inflammation is present due t ...
s and athlete's foot
Athlete's foot, known medically as ''tinea pedis'', is a common skin infection of the feet caused by a fungus. Signs and symptoms often include itching, scaling, cracking and redness. In rare cases the skin may blister. Athlete's foot fungus ...
. The advantages of electrochlorination in this process are as follows:
*Not irritating to skin or soft tissue.
*Active in small concentrations.
*Longer lifespan of chemical and therefore less replacement necessary.
*Easily measurable.
References
{{Reflist Electrochemistry Chlorine