copper smelting on:
[Wikipedia]
[Google]
[Amazon]
 Smelting is a process of applying heat to
Smelting is a process of applying heat to

 Smelting involves more than just melting the metal out of its ore. Most ores are the chemical compound of the metal and other elements, such as oxygen (as an
Smelting involves more than just melting the metal out of its ore. Most ores are the chemical compound of the metal and other elements, such as oxygen (as an
 After tin and lead, the next metal smelted appears to have been copper. How the discovery came about is debated. Campfires are about 200 °C short of the temperature needed, so some propose that the first smelting of copper may have occurred in pottery
After tin and lead, the next metal smelted appears to have been copper. How the discovery came about is debated. Campfires are about 200 °C short of the temperature needed, so some propose that the first smelting of copper may have occurred in pottery
, and dates to 930 BCE (
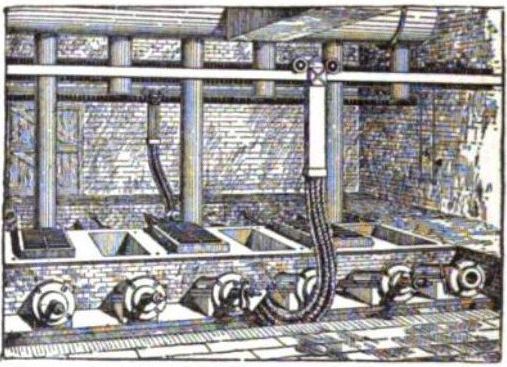 The ores of base metals are often sulfides. In recent centuries,
The ores of base metals are often sulfides. In recent centuries,
 Smelting is a process of applying heat to
Smelting is a process of applying heat to ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 April ...
, to extract a base metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typica ...
. It is a form of extractive metallurgy
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, wash ...
. It is used to extract many metals from their ores, including silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in fro ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish- ...
, and other base metals
A base metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past ...
. Smelting uses heat and a chemical reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth met ...
to decompose the ore, driving off other elements as gases or slag
Slag is a by-product of smelting ( pyrometallurgical) ores and used metals. Broadly, it can be classified as ferrous (by-products of processing iron and steel), ferroalloy (by-product of ferroalloy production) or non-ferrous/base metals (by-pr ...
and leaving the metal base behind. The reducing agent is commonly a fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
source of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes u ...
, such as coke—or, in earlier times, charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, cal ...
. The oxygen in the ore binds to carbon at high temperatures due to the lower potential energy of the bonds in carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is tr ...
(). Smelting most prominently takes place in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric ...
to produce pig iron, which is converted into steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resist ...
.
The carbon source acts as a chemical reactant to remove oxygen from the ore, yielding the purified metal element as a product. The carbon source is oxidized in two stages. First, the carbon (C) combusts with oxygen (O2) in the air to produce carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simp ...
(CO). Second, the carbon monoxide reacts with the ore (e.g. Fe2O3) and removes one of its oxygen atoms, releasing carbon dioxide (). After successive interactions with carbon monoxide, all of the oxygen in the ore will be removed, leaving the raw metal element (e.g. Fe). As most ores are impure, it is often necessary to use a flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications to physics. For transport p ...
, such as limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms wh ...
(or dolomite), to remove the accompanying rock gangue
In mining, gangue () is the commercially worthless material that surrounds, or is closely mixed with, a wanted mineral in an ore deposit. It is thus distinct from overburden, which is the waste rock or materials overlying an ore or mineral body t ...
as slag. This calcination
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), gene ...
reaction also frequently emits carbon dioxide.
Plants for the electrolytic
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
reduction of aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ...
are also generally referred to as aluminium smelters.
Process

 Smelting involves more than just melting the metal out of its ore. Most ores are the chemical compound of the metal and other elements, such as oxygen (as an
Smelting involves more than just melting the metal out of its ore. Most ores are the chemical compound of the metal and other elements, such as oxygen (as an oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of th ...
), sulfur (as a sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
), or carbon and oxygen together (as a carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
). To extract the metal, workers must make these compounds undergo a chemical reaction. Smelting therefore consists of using suitable reducing substances that combine with those oxidizing
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
elements to free the metal.
Roasting
In the case of sulfides and carbonates, a process called "roasting
Roasting is a cooking method that uses dry heat where hot air covers the food, cooking it evenly on all sides with temperatures of at least from an open flame, oven, or other heat source. Roasting can enhance the flavor through caramelizatio ...
" removes the unwanted carbon or sulfur, leaving an oxide, which can be directly reduced. Roasting is usually carried out in an oxidizing environment. A few practical examples:
* Malachite
Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures ...
, a common ore of copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish- ...
, is primarily copper carbonate hydroxide Cu2(CO3)(OH)2. This mineral undergoes thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
to 2CuO, CO2, and H2O in several stages between 250 °C and 350 °C. The carbon dioxide and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
are expelled into the atmosphere, leaving copper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It i ...
, which can be directly reduced to copper as described in the following section titled ''Reduction''.
* Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
, the most common mineral of lead, is primarily lead sulfide (PbS). The sulfide is oxidized to a sulfite (PbSO3), which thermally decomposes into lead oxide and sulfur dioxide gas. (PbO and SO2) The sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ac ...
is expelled (like the carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is tr ...
in the previous example), and the lead oxide is reduced as below.
Reduction
Reduction is the final, high-temperature step in smelting, in which the oxide becomes the elemental metal. A reducing environment (often provided by carbon monoxide, made by incomplete combustion in an air-starved furnace) pulls the finaloxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
atoms from the raw metal. The required temperature varies over a very large range, both in absolute terms and in terms of the melting point of the base metal. Examples:
* Iron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which ...
becomes metallic iron at roughly 1250 °C (2282 °F or 1523.15 K), almost 300 degrees ''below'' iron's melting point of 1538 °C (2800.4 °F or 1811.15 K).
* Mercuric oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is ...
becomes vaporous mercury near 550 °C (1022 °F or 823.15 K), almost 600 degrees ''above'' mercury's melting point of -38 °C (-36.4 °F or 235.15 K).
Flux and slag can provide a secondary service after the reduction step is complete: they provide a molten cover on the purified metal, preventing contact with oxygen while still hot enough to readily oxidize. This prevents impurities from forming in the metal.
Fluxes
Metal workers use fluxes in smelting for several purposes, chief among them catalyzing the desired reactions and chemically binding to unwanted impurities or reaction products. Calcium oxide, in the form oflime
Lime commonly refers to:
* Lime (fruit), a green citrus fruit
* Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide
* Lime (color), a color between yellow and green
Lime may also refer to:
Botany
...
, was often used for this purpose, since it could react with the carbon dioxide and sulfur dioxide produced during roasting and smelting to keep them out of the working environment.
History
Of the seven metals known in antiquity, onlygold
Gold is a chemical element with the Symbol (chemistry), symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a Brightness, bright, slightly orange-yellow, dense, s ...
occurs regularly in native form in the natural environment. The others – copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish- ...
, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, l ...
, silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in fro ...
and mercury – occur primarily as minerals, though copper is occasionally found in its native state
In biochemistry, the native state of a protein or nucleic acid is its properly folded and/or assembled form, which is operative and functional. The native state of a biomolecule may possess all four levels of biomolecular structure, with the ...
in commercially significant quantities. These minerals are primarily carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
s, sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
s, or oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of th ...
s of the metal, mixed with other components such as silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
and alumina. Roasting
Roasting is a cooking method that uses dry heat where hot air covers the food, cooking it evenly on all sides with temperatures of at least from an open flame, oven, or other heat source. Roasting can enhance the flavor through caramelizatio ...
the carbonate and sulfide minerals in air converts them to oxides. The oxides, in turn, are smelted into the metal. Carbon monoxide was (and is) the reducing agent of choice for smelting. It is easily produced during the heating process, and as a gas comes into intimate contact with the ore.
In the Old World
The "Old World" is a term for Afro-Eurasia that originated in Europe , after Europeans became aware of the existence of the Americas. It is used to contrast the continents of Africa, Europe, and Asia, which were previously thought of by th ...
, humans learned to smelt metals in prehistoric
Prehistory, also known as pre-literary history, is the period of human history between the use of the first stone tools by hominins 3.3 million years ago and the beginning of recorded history with the invention of writing systems. The use o ...
times, more than 8000 years ago. The discovery and use of the "useful" metals – copper and bronze at first, then iron a few millennia later – had an enormous impact on human society. The impact was so pervasive that scholars traditionally divide ancient history into Stone Age
The Stone Age was a broad prehistoric period during which stone was widely used to make tools with an edge, a point, or a percussion surface. The period lasted for roughly 3.4 million years, and ended between 4,000 BC and 2,000 BC, with th ...
, Bronze Age
The Bronze Age is a historic period, lasting approximately from 3300 BC to 1200 BC, characterized by the use of bronze, the presence of writing in some areas, and other early features of urban civilization. The Bronze Age is the second pr ...
, and Iron Age
The Iron Age is the final epoch of the three-age division of the prehistory and protohistory of humanity. It was preceded by the Stone Age ( Paleolithic, Mesolithic, Neolithic) and the Bronze Age ( Chalcolithic). The concept has been mostl ...
.
In the Americas
The Americas, which are sometimes collectively called America, are a landmass comprising the totality of North and South America. The Americas make up most of the land in Earth's Western Hemisphere and comprise the New World.
Along with t ...
, pre- Inca civilizations of the central Andes
The Andes, Andes Mountains or Andean Mountains (; ) are the longest continental mountain range in the world, forming a continuous highland along the western edge of South America. The range is long, wide (widest between 18°S – 20°S l ...
in Peru had mastered the smelting of copper and silver at least six centuries before the first Europeans arrived in the 16th century, while never mastering the smelting of metals such as iron for use with weapon-craft.
Tin and lead
In theOld World
The "Old World" is a term for Afro-Eurasia that originated in Europe , after Europeans became aware of the existence of the Americas. It is used to contrast the continents of Africa, Europe, and Asia, which were previously thought of by th ...
, the first metals smelted were tin and lead. The earliest known cast lead beads were found in the Çatalhöyük
Çatalhöyük (; also ''Çatal Höyük'' and ''Çatal Hüyük''; from Turkish ''çatal'' "fork" + ''höyük'' " tumulus") is a tell of a very large Neolithic and Chalcolithic proto-city settlement in southern Anatolia, which existed from ...
site in Anatolia
Anatolia, tr, Anadolu Yarımadası), and the Anatolian plateau, also known as Asia Minor, is a large peninsula in Western Asia and the westernmost protrusion of the Asian continent. It constitutes the major part of modern-day Turkey. The re ...
(Turkey
Turkey ( tr, Türkiye ), officially the Republic of Türkiye ( tr, Türkiye Cumhuriyeti, links=no ), is a transcontinental country located mainly on the Anatolian Peninsula in Western Asia, with a small portion on the Balkan Peninsula in ...
), and dated from about 6500 BC, but the metal may have been known earlier.
Since the discovery happened several millennia before the invention of writing, there is no written record about how it was made. However, tin and lead can be smelted by placing the ores in a wood fire, leaving the possibility that the discovery may have occurred by accident.
Lead is a common metal, but its discovery had relatively little impact in the ancient world. It is too soft to use for structural elements or weapons, though its high density relative to other metals makes it ideal for sling projectiles. However, since it was easy to cast and shape, workers in the classical world of Ancient Greece
Ancient Greece ( el, Ἑλλάς, Hellás) was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity ( AD 600), that comprised a loose collection of cult ...
and Ancient Rome
In modern historiography, ancient Rome refers to Roman civilisation from the founding of the city of Rome in the 8th century BC to the collapse of the Western Roman Empire in the 5th century AD. It encompasses the Roman Kingdom (753–509 ...
used it extensively to pipe and store water. They also used it as a mortar in stone buildings.
Tin was much less common than lead and is only marginally harder, and had even less impact by itself.
Copper and bronze
 After tin and lead, the next metal smelted appears to have been copper. How the discovery came about is debated. Campfires are about 200 °C short of the temperature needed, so some propose that the first smelting of copper may have occurred in pottery
After tin and lead, the next metal smelted appears to have been copper. How the discovery came about is debated. Campfires are about 200 °C short of the temperature needed, so some propose that the first smelting of copper may have occurred in pottery kiln
A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or chemical changes. Kilns have been used for millennia to turn objects made from clay int ...
s. The development of copper smelting in the Andes, which is believed to have occurred independently of the Old World
The "Old World" is a term for Afro-Eurasia that originated in Europe , after Europeans became aware of the existence of the Americas. It is used to contrast the continents of Africa, Europe, and Asia, which were previously thought of by th ...
, may have occurred in the same way. The earliest current evidence of copper smelting, dating from between 5500 BC and 5000 BC, has been found in Pločnik and Belovode, Serbia. A mace head found in Can Hasan, Turkey and dated to 5000 BC, once thought to be the oldest evidence, now appears to be hammered native copper.
Combining copper with tin and/or arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
in the right proportions produces bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
, an alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
that is significantly harder than copper. The first copper/arsenic bronzes date from 4200 BC from Asia Minor
Anatolia, tr, Anadolu Yarımadası), and the Anatolian plateau, also known as Asia Minor, is a large peninsula in Western Asia and the westernmost protrusion of the Asian continent. It constitutes the major part of modern-day Turkey. The re ...
. The Inca bronze alloys were also of this type. Arsenic is often an impurity in copper ores, so the discovery could have been made by accident. Eventually arsenic-bearing minerals were intentionally added during smelting.
Copper–tin bronzes, harder and more durable, were developed around 3500 BC, also in Asia Minor.
How smiths learned to produce copper/tin bronzes is unknown. The first such bronzes may have been a lucky accident from tin-contaminated copper ores. However, by 2000 BC, people were mining tin on purpose to produce bronze—which is amazing given that tin is a semi-rare metal, and even a rich cassiterite
Cassiterite is a tin oxide mineral, SnO2. It is generally opaque, but it is translucent in thin crystals. Its luster and multiple crystal faces produce a desirable gem. Cassiterite was the chief tin ore throughout ancient history and remains ...
ore only has 5% tin. Also, it takes special skills (or special instruments) to find it and locate richer lode
In geology, a lode is a deposit of metalliferous ore that fills or is embedded in a fissure (or crack) in a rock formation or a vein of ore that is deposited or embedded between layers of rock.
The current meaning (ore vein) dates from the 1 ...
s. However early peoples learned about tin, they understood how to use it to make bronze by 2000 BC.
The discovery of copper and bronze manufacture had a significant impact on the history of the Old World
The "Old World" is a term for Afro-Eurasia that originated in Europe , after Europeans became aware of the existence of the Americas. It is used to contrast the continents of Africa, Europe, and Asia, which were previously thought of by th ...
. Metals were hard enough to make weapons that were heavier, stronger, and more resistant to impact damage than wood, bone, or stone equivalents. For several millennia, bronze was the material of choice for weapons such as sword
A sword is an edged, bladed weapon intended for manual cutting or thrusting. Its blade, longer than a knife or dagger, is attached to a hilt and can be straight or curved. A thrusting sword tends to have a straighter blade with a pointed ti ...
s, dagger
A dagger is a fighting knife with a very sharp point and usually two sharp edges, typically designed or capable of being used as a thrusting or stabbing weapon.State v. Martin, 633 S.W.2d 80 (Mo. 1982): This is the dictionary or popular-use de ...
s, battle axe
A battle axe (also battle-axe, battle ax, or battle-ax) is an axe specifically designed for combat. Battle axes were specialized versions of utility axes. Many were suitable for use in one hand, while others were larger and were deployed two-h ...
s, and spear
A spear is a pole weapon consisting of a shaft, usually of wood, with a pointed head. The head may be simply the sharpened end of the shaft itself, as is the case with fire hardened spears, or it may be made of a more durable material fasten ...
and arrow
An arrow is a fin-stabilized projectile launched by a bow. A typical arrow usually consists of a long, stiff, straight shaft with a weighty (and usually sharp and pointed) arrowhead attached to the front end, multiple fin-like stabilizers ca ...
points, as well as protective gear such as shield
A shield is a piece of personal armour held in the hand, which may or may not be strapped to the wrist or forearm. Shields are used to intercept specific attacks, whether from close-ranged weaponry or projectiles such as arrows, by means of a ...
s, helmet
A helmet is a form of protective gear worn to protect the head. More specifically, a helmet complements the skull in protecting the human brain. Ceremonial or symbolic helmets (e.g., a policeman's helmet in the United Kingdom) without protect ...
s, greave
A greave (from the Old French ''greve'' "shin, shin armour") or jambeau is a piece of armour that protects the leg.
Description
The primary purpose of greaves is to protect the tibia from attack. The tibia, or shinbone, is very close to the ski ...
s (metal shin guards), and other body armor
Body armor, also known as body armour, personal armor or armour, or a suit or coat of armor, is protective clothing designed to absorb or deflect physical attacks. Historically used to protect military personnel, today it is also used by variou ...
. Bronze also supplanted stone, wood, and organic materials in tools and household utensils—such as chisel
A chisel is a tool with a characteristically shaped cutting edge (such that wood chisels have lent part of their name to a particular Grind#Typical grinds, grind) of blade on its end, for carving or cutting a hard material such as wood, Rock (g ...
s, saw
A saw is a tool consisting of a tough blade, wire, or chain with a hard toothed edge. It is used to cut through material, very often wood, though sometimes metal or stone. The cut is made by placing the toothed edge against the material and mo ...
s, adze
An adze (; alternative spelling: adz) is an ancient and versatile cutting tool similar to an axe but with the cutting edge perpendicular to the handle rather than parallel. Adzes have been used since the Stone Age. They are used for smoothing ...
s, nails, blade shears
A blade is the portion of a tool, weapon, or machine with an edge that is designed to puncture, chop, slice or scrape surfaces or materials. Blades are typically made from materials that are harder than those they are to be used on. Histor ...
, knives
A knife ( : knives; from Old Norse 'knife, dirk') is a tool or weapon with a cutting edge or blade, usually attached to a handle or hilt. One of the earliest tools used by humanity, knives appeared at least 2.5 million years ago, as evidence ...
, sewing needle
A sewing needle, used for hand-sewing, is a long slender tool with a pointed tip at one end and a hole (or ''eye'') to hold the sewing thread. The earliest needles were made of bone or wood; modern needles are manufactured from high carbon steel ...
s and pins, jug
A jug is a type of container commonly used to hold liquids. It has an opening, sometimes narrow, from which to pour or drink, and has a handle, and often a pouring lip. Jugs throughout history have been made of metal, and ceramic, or glass, and ...
s, cooking pot
Cookware and bakeware is food preparation equipment, such as cooking pots, pans, baking sheets etc. used in kitchens. Cookware is used on a stove or range cooktop, while bakeware is used in an oven. Some utensils are considered both cookware ...
s and cauldron
A cauldron (or caldron) is a large pot (kettle) for cooking or boiling over an open fire, with a lid and frequently with an arc-shaped hanger and/or integral handles or feet. There is a rich history of cauldron lore in religion, mythology, and ...
s, mirror
A mirror or looking glass is an object that Reflection (physics), reflects an image. Light that bounces off a mirror will show an image of whatever is in front of it, when focused through the lens of the eye or a camera. Mirrors reverse the ...
s, and horse harness
Horse harness is a device that connects a horse to a vehicle or another type of load.
There are two main categories of horse harness: (1) the "breaststrap" or "breastcollar" design, and (2) the collar and hames design. For light work, such as ho ...
es. Tin and copper also contributed to the establishment of trade networks that spanned large areas of Europe and Asia, and had a major effect on the distribution of wealth among individuals and nations.
Early iron smelting
The earliest evidence for iron-making is a small number of iron fragments with the appropriate amounts of carbon admixture found in the Proto-Hittite layers at Kaman-Kalehöyük and dated to 2200–2000 BCE. Souckova-Siegolová (2001) shows that iron implements were made in Central Anatolia in very limited quantities around 1800 BCE and were in general use by elites, though not by commoners, during the New Hittite Empire (∼1400–1200 BCE). Archaeologists have found indications of iron working in Ancient Egypt, somewhere between theThird Intermediate Period
The Third Intermediate Period of ancient Egypt began with the death of Pharaoh Ramesses XI in 1077 BC, which ended the New Kingdom, and was eventually followed by the Late Period. Various points are offered as the beginning for the latte ...
and 23rd Dynasty
The Twenty-third Dynasty of Egypt (notated Dynasty XXIII, alternatively 23rd Dynasty or Dynasty 23) is usually classified as the third dynasty of the ancient Egyptian Third Intermediate Period. This dynasty consisted of a number of Meshwesh ki ...
(ca. 1100–750 BCE). Significantly though, they have found no evidence for iron ore smelting in any (pre-modern) period. In addition, very early instances of carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
were in production around 2000 years ago (around the first century CE.) in northwest Tanzania
Tanzania (; ), officially the United Republic of Tanzania ( sw, Jamhuri ya Muungano wa Tanzania), is a country in East Africa within the African Great Lakes region. It borders Uganda to the north; Kenya to the northeast; Comoro Islands an ...
, based on complex preheating principles. These discoveries are significant for the history of metallurgy.
Most early processes in Europe and Africa involved smelting iron ore in a bloomery
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom''. ...
, where the temperature is kept low enough so that the iron does not melt. This produces a spongy mass of iron called a bloom, which then must be consolidated with a hammer to produce wrought iron. The earliest evidence to date for the bloomery smelting of iron is found at Tell Hammeh, Jordan, and dates to 930 BCE (
C14 dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
The method was de ...
).
Later iron smelting
From the medieval period, an indirect process began to replace direct reduction in bloomeries. This used ablast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric ...
to make pig iron, which then had to undergo a further process to make forgeable bar iron. Processes for the second stage include fining in a finery forge
A finery forge is a forge used to produce wrought iron from pig iron by decarburization in a process called "fining" which involved liquifying cast iron in a fining hearth and removing carbon from the molten cast iron through oxidation. Finery ...
and, from the Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
, puddling. Both processes are now obsolete, and wrought iron is now rarely made. Instead, mild steel is produced from a bessemer converter
The Bessemer process was the first inexpensive industrial process for the mass production of steel from molten pig iron before the development of the open hearth furnace. The key principle is removal of impurities from the iron by oxidation wit ...
or by other means including smelting reduction processes such as the Corex Process.
Base metals
 The ores of base metals are often sulfides. In recent centuries,
The ores of base metals are often sulfides. In recent centuries, reverberatory furnace
A reverberatory furnace is a metallurgical or process furnace that isolates the material being processed from contact with the fuel, but not from contact with combustion gases. The term ''reverberation'' is used here in a generic sense of ''re ...
s have been used to keep the charge being smelted separate from the fuel. Traditionally, they were used for the first step of smelting: forming two liquids, one an oxide slag containing most of the impurities, and the other a sulfide matte containing the valuable metal sulfide and some impurities. Such "reverb" furnaces are today about 40 meters long, 3 meters high and 10 meters wide. Fuel is burned at one end to melt the dry sulfide concentrates (usually after partial roasting) which are fed through openings in the roof of the furnace. The slag floats over the heavier matte and is removed and discarded or recycled. The sulfide matte is then sent to the converter. The precise details of the process vary from one furnace to another depending on the mineralogy of the orebody.
While reverberatory furnaces produced slags containing very little copper, they were relatively energy inefficient and off-gassed a low concentration of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ac ...
that was difficult to capture; a new generation of copper smelting technologies has supplanted them. More recent furnaces exploit bath smelting, top-jetting lance smelting, flash smelting
lang=en, upright=1.3, Development of flash smelting in the copper industry, related to the number of smelters using this technology.
Flash smelting ( fi, Liekkisulatus, literally "flame-smelting") is a smelting process for sulfur-containing ore ...
and blast furnaces. Some examples of bath smelters include the Noranda furnace, the Isasmelt furnace, the Teniente reactor, the Vunyukov smelter and the SKS technology. Top-jetting lance smelters include the Mitsubishi smelting reactor. Flash smelters account for over 50% of the world's copper smelters. There are many more varieties of smelting processes, including the Kivset, Ausmelt, Tamano, EAF, and BF.
Environmental and occupational health impacts
Smelting has serious effects on the environment, producingwastewater
Wastewater is water generated after the use of freshwater, raw water, drinking water or saline water in a variety of deliberate applications or processes. Another definition of wastewater is "Used water from any combination of domestic, industri ...
and slag and releasing such toxic metals as copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish- ...
, silver, iron, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
and selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
into the atmosphere. Smelters also release gaseous sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ac ...
, contributing to acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but ...
, which acidifies soil and water.
The smelter in Flin Flon, Canada was one of the largest point sources of mercury in North America in the 20th century. Even after smelter releases were drastically reduced, landscape re-emission continued to be a major regional source of mercury. Lakes will likely receive mercury contamination from the smelter for decades, from both re-emissions returning as rainwater and leaching of metals from the soil.
Air pollution
Air pollutants generated byaluminum smelter
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has ...
s include carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be conside ...
, hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
, polycyclic compound
In the field of organic chemistry, a polycyclic compound is an organic compound featuring several closed rings of atoms, primarily carbon. These ring substructures include cycloalkanes, aromatics, and other ring types. They come in sizes of ...
s, lead, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
, manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial all ...
, polychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by t ...
s and mercury. Copper smelter emissions include arsenic, beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
, cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
, chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and ha ...
, lead, manganese and nickel. Lead smelters typically emit arsenic, antimony, cadmium and various lead compounds.
Wastewater
Wastewater pollutants discharged by iron and steel mills includes gasification products such asbenzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen a ...
, naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
, anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colo ...
, cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
, ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous w ...
, phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it re ...
s and cresol
Cresols (also hydroxytoluene or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also categorized as methylphenols. ...
s, together with a range of more complex organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The s ...
s known collectively as polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. ...
s (PAH). Treatment technologies include recycling of wastewater; settling basin
A settling basin, settling pond or decant pond is an earthen or concrete structure using sedimentation to remove settleable matter and turbidity from wastewater. The basins are used to control water pollution in diverse industries such as agricul ...
s, clarifier
Clarifiers are settling tanks built with mechanical means for continuous removal of solids being deposited by sedimentation. A clarifier is generally used to remove solid particulates or suspended solids from liquid for clarification and/or thi ...
s and filtration systems for solids removal; oil skimmers and filtration; chemical precipitation and filtration for dissolved metals; carbon adsorption and biological oxidation for organic pollutants; and evaporation.
Pollutants generated by other types of smelters varies with the base metal ore. For example, aluminum smelters typically generate fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ty ...
, benzo(a)pyrene
Benzo 'a''yrene (B''a''P or B ) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between and . The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, espec ...
, antimony and nickel, as well as aluminum. Copper smelters typically discharge cadmium, lead, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
, arsenic and nickel, in addition to copper.
Health impacts
Labourers working in the smelting industry have reported respiratory illnesses inhibiting their ability to perform the physical tasks demanded by their jobs.Regulations
In the United States, theEnvironmental Protection Agency
A biophysical environment is a biotic and abiotic surrounding of an organism or population, and consequently includes the factors that have an influence in their survival, development, and evolution. A biophysical environment can vary in scal ...
has published pollution control regulations for smelters.
* Air pollution standards under the Clean Air Act
* Water pollution standards (effluent guidelines Effluent Guidelines (also referred to as Effluent Limitation Guidelines (ELGs)) are U.S. national standards for wastewater discharges to surface waters and publicly owned treatment works (POTW) (also called municipal sewage treatment plants). The Un ...
) under the Clean Water Act
The Clean Water Act (CWA) is the primary federal law in the United States governing water pollution. Its objective is to restore and maintain the chemical, physical, and biological integrity of the nation's waters; recognizing the responsibilit ...
.
See also
*Cast iron
Cast iron is a class of iron–carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impuriti ...
* Ellingham diagram
An Ellingham diagram is a graph showing the temperature dependence of the stability of compounds. This analysis is usually used to evaluate the ease of reduction of metal oxides and sulfides. These diagrams were first constructed by Harold Elli ...
, useful in predicting the conditions under which an ore reduces to its metal
* Copper extraction techniques
Copper extraction refers to the methods used to obtain copper from its ores. The conversion of copper consists of a series of physical and electrochemical processes. Methods have evolved and vary with country depending on the ore source, loc ...
* Clinker
*Cupellation
Cupellation is a refining process in metallurgy where ores or alloyed metals are treated under very high temperatures and have controlled operations to separate noble metals, like gold and silver, from base metals, like lead, copper, zinc, arsen ...
*Lead smelting
Plants for the production of lead are generally referred to as lead smelters. Primary lead production begins with sintering. Concentrated lead ore is fed into a sintering machine with iron, silica, limestone fluxes, coke, soda ash, pyrite, zinc, ...
*Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the s ...
*Pyrometallurgy
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable ...
* Wrought iron
*Zinc smelting
Zinc smelting is the process of converting zinc concentrates (ores that contain zinc) into pure zinc. Zinc smelting has historically been more difficult than the smelting of other metals, e.g. iron, because in contrast, zinc has a low boiling point ...
References
Bibliography
*Pleiner, R. (2000) ''Iron in Archaeology. The European Bloomery Smelters'', Praha, Archeologický Ústav Av Cr. *Veldhuijzen, H.A. (2005) Technical Ceramics in Early Iron Smelting. The Role of Ceramics in the Early First Millennium Bc Iron Production at Tell Hammeh (Az-Zarqa), Jordan. In: Prudêncio, I.Dias, I. and Waerenborgh, J.C. (Eds.) ''Understanding People through Their Pottery; Proceedings of the 7th European Meeting on Ancient Ceramics (Emac '03)''. Lisboa, Instituto Português de Arqueologia (IPA). *Veldhuijzen, H.A. and Rehren, Th. (2006) Iron Smelting Slag Formation at Tell Hammeh (Az-Zarqa), Jordan. In: Pérez-Arantegui, J. (Ed.) ''Proceedings of the 34th International Symposium on Archaeometry, Zaragoza, 3–7 May 2004''. Zaragoza, Institución «Fernando el Católico» (C.S.I.C.) Excma. Diputación de Zaragoza.External links
{{Authority control Firing techniques Metallurgical processes de:Verhüttung