calcium cycle on:
[Wikipedia]
[Google]
[Amazon]
The calcium cycle is a transfer of calcium between dissolved and



 With
With
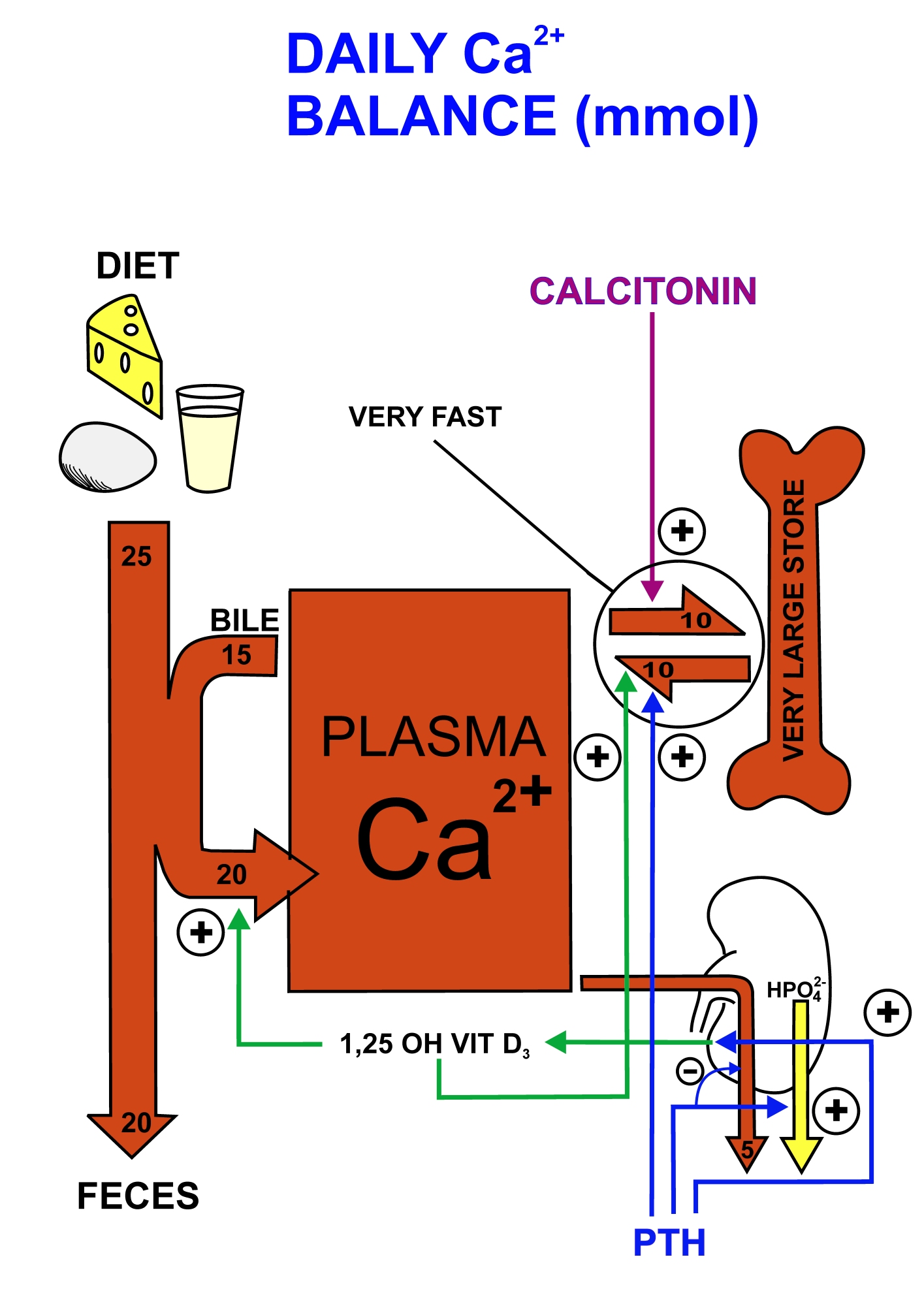 Being an essential element, calcium is obtained through dietary sources, the majority of which comes from dairy products. The most significant mechanisms controlling calcium use within the body are intestinal absorption, renal filtration, renal reabsorption and bone turnover, which are controlled predominantly by hormones and their corresponding receptors in the gut, kidneys and bones respectively. This allows for calcium use throughout the body, namely in
Being an essential element, calcium is obtained through dietary sources, the majority of which comes from dairy products. The most significant mechanisms controlling calcium use within the body are intestinal absorption, renal filtration, renal reabsorption and bone turnover, which are controlled predominantly by hormones and their corresponding receptors in the gut, kidneys and bones respectively. This allows for calcium use throughout the body, namely in
 Calcium is an essential component of soil. When deposited in the form of lime, it cannot be used by plants. To combat this, carbon dioxide produced by plants reacts with water in the environment to produce carbonic acid. Carbonic acid is then able to dissolve limestone, enabling the release of calcium ions. This reaction is more readily available with smaller particles of limestone than it is with large pieces of rock due to the increased surface area. When lime is leached into soil, calcium levels inevitably increase, both stabilising pH and enabling calcium to mix with water to form Ca2+ ions, thus making it soluble and accessible to plants to be absorbed and utilised by the root system. The calcium ions travel up the xylem of the plant alongside water to reach the leaves. The plant can utilise this calcium in the form of calcium pectate to stabilise cell walls and provide rigidity. Calcium is also used by plant enzymes to signal growth and coordinate life-promoting processes. Additionally, the release of calcium ions enables microorganisms to access phosphorus and other micro nutrients with greater ease, improving the soil ecosystem drastically thus indirectly promoting plant growth and nutrition.
Inevitable plant and animal death results in the return of calcium contained within the organism back into the soil to be utilised by other plants. Decomposing organisms break them down, returning the calcium back into the soil and enabling the cycling of calcium to continue. Additionally, these animals and plants are eaten by other animals, similarly continuing the cycle. It is however important to note the modern introduction of calcium into the soil by humans (through fertilisers and other horticultural products) has resulted in a higher concentration of calcium contained within soil.
Calcium is an essential component of soil. When deposited in the form of lime, it cannot be used by plants. To combat this, carbon dioxide produced by plants reacts with water in the environment to produce carbonic acid. Carbonic acid is then able to dissolve limestone, enabling the release of calcium ions. This reaction is more readily available with smaller particles of limestone than it is with large pieces of rock due to the increased surface area. When lime is leached into soil, calcium levels inevitably increase, both stabilising pH and enabling calcium to mix with water to form Ca2+ ions, thus making it soluble and accessible to plants to be absorbed and utilised by the root system. The calcium ions travel up the xylem of the plant alongside water to reach the leaves. The plant can utilise this calcium in the form of calcium pectate to stabilise cell walls and provide rigidity. Calcium is also used by plant enzymes to signal growth and coordinate life-promoting processes. Additionally, the release of calcium ions enables microorganisms to access phosphorus and other micro nutrients with greater ease, improving the soil ecosystem drastically thus indirectly promoting plant growth and nutrition.
Inevitable plant and animal death results in the return of calcium contained within the organism back into the soil to be utilised by other plants. Decomposing organisms break them down, returning the calcium back into the soil and enabling the cycling of calcium to continue. Additionally, these animals and plants are eaten by other animals, similarly continuing the cycle. It is however important to note the modern introduction of calcium into the soil by humans (through fertilisers and other horticultural products) has resulted in a higher concentration of calcium contained within soil.
 With its widespread uses, a large volume of calcium must be obtained from mines and quarries to supply the high demand. As more limestone and water is removed from mines, underground stores of rock are often weakened making the ground more susceptible to sink holes. Sinkholes and mining both affect the presence of groundwater, potentially leading to a lower water table or altered pathways of flowing water. This may affect local ecosystems or farmland as the water supply is restricted. Additionally, the water that is released from mining areas will have higher concentrations of dissolved calcium. This can either be released into oceans or absorbed by the soil. Whilst not always detrimental, it alters the natural calcium cycle which may have flow-on effects for ecosystems. Furthermore, water being pumped from mines increases the danger of downstream flooding whilst simultaneously decreasing the volume on water in upstream reservoirs such as marshes, ponds of wetlands It is however important to note than limestone mining is comparatively less damaging than other mining process, with potential to restore the environment after the mine is no longer in use
With its widespread uses, a large volume of calcium must be obtained from mines and quarries to supply the high demand. As more limestone and water is removed from mines, underground stores of rock are often weakened making the ground more susceptible to sink holes. Sinkholes and mining both affect the presence of groundwater, potentially leading to a lower water table or altered pathways of flowing water. This may affect local ecosystems or farmland as the water supply is restricted. Additionally, the water that is released from mining areas will have higher concentrations of dissolved calcium. This can either be released into oceans or absorbed by the soil. Whilst not always detrimental, it alters the natural calcium cycle which may have flow-on effects for ecosystems. Furthermore, water being pumped from mines increases the danger of downstream flooding whilst simultaneously decreasing the volume on water in upstream reservoirs such as marshes, ponds of wetlands It is however important to note than limestone mining is comparatively less damaging than other mining process, with potential to restore the environment after the mine is no longer in use
solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
phases. There is a continuous supply of calcium ions
Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction ...
into waterways from rocks
In geology, rock (or stone) is any naturally occurring solid mass or aggregate of minerals or mineraloid matter. It is categorized by the minerals included, its chemical composition, and the way in which it is formed. Rocks form the Earth's ...
, organism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s, and soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
and calcium silicate, which can deposit to form sediments or the exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
s of organisms. Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s and teeth
A tooth (: teeth) is a hard, calcified structure found in the jaws (or mouths) of many vertebrates and used to break down food. Some animals, particularly carnivores and omnivores, also use teeth to help with capturing or wounding prey, tear ...
or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of three main greenhouse gases in the atmosphere of Earth. The concen ...
due to ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
.
Calcium weathering and inputs to seawater
Calcium is stored in geologic reservoirs, most commonly in the form ofcalcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
or as calcium silicate. Calcium-containing rocks include calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, dolomite, phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
, and gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
. Rocks slowly dissolve by physical and chemical processes, carrying calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
ions into rivers and oceans. Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
ions (Ca2+) and magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
ions (Mg2+) have the same charge (+2) and similar sizes, so they react similarly and are able to substitute for each other in some minerals, such as carbonates
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . ...
. Ca2+-containing minerals are often more easily weathered than Mg2+ minerals, so Ca2+ is often more enriched in waterways than Mg2+. Rivers containing more dissolved Ca2+ are generally considered more alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The ...
.
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
is one of the most common elements found in seawater. Inputs of dissolved calcium (Ca2+) to the ocean include the weathering of calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
, calcium silicate, and calcium carbonate, basalt-seawater reaction, and dolomitization
Dolomitization is a geological process where magnesium ions replace calcium ions in the mineral calcite, resulting in the formation of dolomite.
Dolomitization conditions are present in Abu Dhabi, the Mediterranean Sea, and some Brazilian hyp ...
.
Biogenic calcium carbonate and the biological pump
Biogenic calcium carbonate is formed when marine organisms, such ascoccolithophore
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom ...
s, coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s, pteropods, and other mollusks
Mollusca is a phylum of protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant species of molluscs are recognized, making it the second-largest animal phylum after Arthropoda. The num ...
transform calcium ions and bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
into shells and exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
s of calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
or aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
, both forms of calcium carbonate. This is the dominant sink for dissolved calcium in the ocean. Dead organisms sink to the bottom of the ocean, depositing layers of shell which over time cement to form limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
. This is the origin of both marine and terrestrial limestone.
Calcium precipitates into calcium carbonate according to the following equation:
Ca2+ + 2HCO3− → CO2+ H2O + CaCO3
The relationship between dissolved calcium and calcium carbonate is affected greatly by the levels of carbon dioxide (CO2) in the atmosphere.
Increased carbon dioxide leads to more bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
in the ocean according to the following equation:
CO2 + CO32− + H2O → 2HCO3−



 With
With ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
, inputs of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
promote the dissolution of calcium carbonate and harm marine organisms dependent on their protective calcite or aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
shells. The solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
of calcium carbonate increases with pressure and carbon dioxide and decreases with temperature. Thus, calcium carbonate is more soluble in deep waters than surface waters due to higher pressure and lower temperature. As a result, precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwe ...
of calcium carbonate is more common in shallower oceans. The depth at which the rate of calcite dissolution equals the rate of calcite precipitation is known as calcite compensation depth.
Changes in global climate and the calcium cycle
Ocean acidity due to carbon dioxide has already increased by 25% since the industrial revolution. As carbon dioxide emissions continually increase and accumulate, this will negatively affect the lives of many marine ecosystems. The calcium carbonate used to form many marine organisms' exoskeletons will begin to break down, leaving these animals vulnerable and unable to live in their habitats. This ultimately has a flow on effect to predators, further affecting the function of many food webs globally.Changes in calcium concentrations over geologic time
Calcium stable isotopes have been used to study inputs and outputs of dissolved calcium in marine environments. For example, one study found that calcium levels have decreased between 25 and 50 percent over a 40 million year timespan, suggesting that dissolved Ca2+outputs have exceeded its inputs. The isotope Calcium-44 can help to indicate variations in calcium carbonate over long timespans and help explain variants in global temperature. Declines in the isotope Calcium-44 usually correlate with periods of cooling, as dissolution of calcium carbonate typically signifies a decrease in temperature. Thus, Calcium isotopes correlate with Earth's climate over long periods of time.Human/animal use of calcium
 Being an essential element, calcium is obtained through dietary sources, the majority of which comes from dairy products. The most significant mechanisms controlling calcium use within the body are intestinal absorption, renal filtration, renal reabsorption and bone turnover, which are controlled predominantly by hormones and their corresponding receptors in the gut, kidneys and bones respectively. This allows for calcium use throughout the body, namely in
Being an essential element, calcium is obtained through dietary sources, the majority of which comes from dairy products. The most significant mechanisms controlling calcium use within the body are intestinal absorption, renal filtration, renal reabsorption and bone turnover, which are controlled predominantly by hormones and their corresponding receptors in the gut, kidneys and bones respectively. This allows for calcium use throughout the body, namely in bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
growth, cellular signalling
In biology, cell signaling (cell signalling in British English) is the Biological process, process by which a Cell (biology), cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all Cell (biol ...
, blood clotting, muscle contraction and neuron
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural net ...
function.
Calcium is one of the essential components of bone, contributing to its strength and structure in addition to being the main site at which it is stored within the body. Within the muscles, its primary use is to enable contractions. Muscle cells draw calcium from the blood, allowing it to bind with troponin, a component of the muscle fibre that signals for a contraction by moving actin and myosin. After a contraction, calcium dissipates and the filaments move back to a resting state before the release of more calcium for the next contraction. Furthermore, calcium plays a significant role in allowing nerve impulses to be transmitted between neurons. The release of calcium ions from voltage gated ion channels signals for the release of neurotransmitters into the synapse. This allows for the depolarisation of a neuron, thus transmitting the signal to the next neuron where this process is once again repeated. Without the presence of calcium ions, the release of neurotransmitters would not occur, preventing signals from being sent and hindering body processes.
Negative feedback mechanisms are implemented in order to control calcium levels. When low calcium levels are detected in the body, the parathyroid releases parathyroid hormone
Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates serum calcium and phosphate through its actions on the bone, kidneys, and small intestine. PTH incre ...
(PTH) which travels through the bloodstream to the bones and kidneys. In the bones, the presence of PTH stimulates osteoclasts. These cells break down bone to release calcium into the bloodstream where it can be used by the rest of the body in the above processes. In the kidneys, PTH stimulates re-absorption of calcium so it in not lost from the body through urine and returned to the bloodstream instead. Lastly, PTH acts on the intestines by indirectly promoting enzymes that activate vitamin D, a signal for the intestines to absorb more calcium, further increasing blood calcium levels. This will continue until the body releases too much calcium into the bloodstream. Excess calcium then promotes the release of calcitonin from the thyroid gland, effectively reversing the process of PTH. Osteoclast activity is stopped and osteoblasts take over, utilising the excess calcium in the bloodstream to form new bone. Calcium re-absorption in the kidney is prevented, allowing the excretion of excess calcium through the urine. Through these hormonal mechanisms, calcium homeostasis is maintained within the body.
Calcium in plants and soil
Industrial uses of calcium and its impact on the calcium cycle
The naturally occurring calcium cycle has been altered by human intervention. Calcium is predominantly extracted from limestone deposits to be utilised by many industrial processes. Purification of iron ore and aluminium, replacing asbestos brake linings and some coatings for electric cables, are some of these major uses of calcium. Furthermore, calcium is used within the household to maintain alkaline pH of swimming pools, counteracting acidic disinfectants and in the food production industry to produce bicarbonate soda, some wines and dough. With its widespread uses, a large volume of calcium must be obtained from mines and quarries to supply the high demand. As more limestone and water is removed from mines, underground stores of rock are often weakened making the ground more susceptible to sink holes. Sinkholes and mining both affect the presence of groundwater, potentially leading to a lower water table or altered pathways of flowing water. This may affect local ecosystems or farmland as the water supply is restricted. Additionally, the water that is released from mining areas will have higher concentrations of dissolved calcium. This can either be released into oceans or absorbed by the soil. Whilst not always detrimental, it alters the natural calcium cycle which may have flow-on effects for ecosystems. Furthermore, water being pumped from mines increases the danger of downstream flooding whilst simultaneously decreasing the volume on water in upstream reservoirs such as marshes, ponds of wetlands It is however important to note than limestone mining is comparatively less damaging than other mining process, with potential to restore the environment after the mine is no longer in use
With its widespread uses, a large volume of calcium must be obtained from mines and quarries to supply the high demand. As more limestone and water is removed from mines, underground stores of rock are often weakened making the ground more susceptible to sink holes. Sinkholes and mining both affect the presence of groundwater, potentially leading to a lower water table or altered pathways of flowing water. This may affect local ecosystems or farmland as the water supply is restricted. Additionally, the water that is released from mining areas will have higher concentrations of dissolved calcium. This can either be released into oceans or absorbed by the soil. Whilst not always detrimental, it alters the natural calcium cycle which may have flow-on effects for ecosystems. Furthermore, water being pumped from mines increases the danger of downstream flooding whilst simultaneously decreasing the volume on water in upstream reservoirs such as marshes, ponds of wetlands It is however important to note than limestone mining is comparatively less damaging than other mining process, with potential to restore the environment after the mine is no longer in use
The importance of the calcium cycle and future predictions
The calcium cycle links ionic and non ionic calcium together in both marine and terrestrial environments and is essential for the functioning of all living organisms. In animals, calcium enables neurons to transmit signals by opening voltage gated channels that allow neurotransmitters to reach the next cell, bone formation and development and kidney function, whilst being maintained by hormones that ensure calcium homeostasis is reached. In plants, calcium promotes enzyme activity and ensures cell wall function, providing stability to plants. It also enables crustaceans to form shells and corals to exist, as calcium provides structure, rigidity and strength to structures when complexed (combined) to other atoms. Without its presence in the environment, many life-preserving processes would not exist. In the modern context, calcium also enables many industrial processes to occur, promoting further technological developments. With its close relation to thecarbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
and the effects of greenhouse gasses, both calcium and carbon cycles are predicted to change in the coming years. Tracking calcium isotopes enables the prediction of environmental changes, with many sources suggesting increasing temperatures in both the atmosphere and marine environment. As a result, this will drastically alter the breakdown of rock, the pH of oceans and waterways and thus calcium sedimentation, hosting an array of implications on the calcium cycle.
Due to the complex interactions of calcium with many facets of life, the effects of altered environmental conditions are unlikely to be known until they occur. Predictions can however be tentatively made, based upon evidence-based research. Increasing carbon dioxide levels and decreasing ocean pH will alter calcium solubility, preventing corals and shelled organisms from developing their calcium-based exoskeletons, thus making them vulnerable or unable to survive.
References
{{Reflist Biogeochemical cycle Biochemistry