|
Vanadium Pentafluoride
Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid that freezes near room temperature. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances. Properties and structure The compound is exclusively a monomer in the gas phase. In the gas phase it adopts D3h symmetric trigonal bipyramidal geometry as indicated by electron diffraction. As a solid, VF5 forms a polymeric structure with fluoride-bridged octahedral vanadium centers. The formation enthalpy of VF5 is -1429.4 ± 0.8 kJ/mol. Synthesis Vanadium pentafluoride can be prepared by fluorination of vanadium metal: : 2 V + 5 F2 → 2 VF5 Alternatively, disproportionation of vanadium tetrafluoride yields equal amounts of the solid trifluoride and the volatile pentafluoride: :2 VF4 → VF3 + VF5 This conversion is conducted at 650 °C. It can also be synthesized by using elemental fluorine to fluorinate industrial co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium(V) Chloride
Vanadium(V) chloride is the inorganic compound with the formula VCl5. It is a black diamagnetic solid. The molecules adopt a bioctahedral structure similar to that of niobium(V) chloride. Preparation and reactions Chlorine cannot oxidise vanadium(IV); chlorination of vanadium metal will yield only vanadium(IV) chloride. Vanadium(V) chloride is instead prepared from vanadium pentafluoride with excess boron trichloride as a chlorinating agent: : It is unstable at room temperature, releasing gaseous chlorine and giving vanadium(IV) chloride Vanadium tetrachloride is the inorganic compound with the formula V Cl4. This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds. Synthesis, bonding, basic properties With one more valence electron ...: : In contrast, the heavier analogues and are stable and not particularly oxidizing. References {{Chlorides Vanadium(V) compounds Chlorides Metal halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leningrad
Saint Petersburg, formerly known as Petrograd and later Leningrad, is the List of cities and towns in Russia by population, second-largest city in Russia after Moscow. It is situated on the Neva, River Neva, at the head of the Gulf of Finland on the Baltic Sea. The city had a population of 5,601,911 residents as of 2021, with more than 6.4 million people living in the Saint Petersburg metropolitan area, metropolitan area. Saint Petersburg is the List of European cities by population within city limits, fourth-most populous city in Europe, the List of cities and towns around the Baltic Sea, most populous city on the Baltic Sea, and the world's List of northernmost items#Cities and settlements, northernmost city of more than 1 million residents. As the former capital of the Russian Empire, and a Ports of the Baltic Sea, historically strategic port, it is governed as a Federal cities of Russia, federal city. The city was founded by Tsar Peter the Great on 27 May 1703 on the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boris Nikolsky
Boris Petrovich Nikolsky (; – 4 January 1990), , was a Soviet chemist who played a crucial role in the former Soviet program of nuclear weapons. Besides his work on the plutonium chemistry, Nikolsky did a pioneering work in ion exchanges applications in radiochemistry and physical chemistry, and was a professor of chemistry at the Leningrad University (now Saint Petersburg State University). He academician of the Soviet Academy of Sciences. Boris Nikolsky was a 1925 graduate of Leningrad State University. In the 1930s he studied the ion exchange processes between aqueous solutions and solids. During that time Nikolsky developed the theory of ion exchange in glass electrodes. He derived equations that describe properties of glass electrodes as well as other types of ion-selective electrodes depending on chemical structure and multi-component composition of glass, concurrent interference of ions (see Nikolsky-Eisenman equation and Nikolsky- Shultz-Eisenman thermodynamic ion-e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyhalide
In chemistry, oxohalides or oxyhalides are a group of chemical compounds with the chemical formula , where X is a halogen, and A is an element different than O and X. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I). The element A may be a main group element, a transition element, a rare earth element or an actinide. Molecular oxohalides are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. The term ''oxohalide'', or ''oxyhalide'', also refers to ionic oxohalides with the same overall chemical formula, but having an ionic structure. There are minerals that are ionic oxohalides. Synthesis Oxohalides can be seen as compounds intermediate between oxides and halides. There are three general methods of synthesis: *Partial oxidation of a halide: *: **In this example, the oxidation state increases by two and the electrical charge is unchanged. *Partial halogenation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
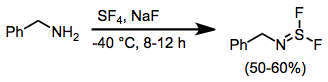
Sulfur Tetrafluoride
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physicochemical
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to understand include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of cell membr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosive Substance
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductivity (electrolytic)
Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter (S/m). Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. In many cases, conductivity is linked directly to the total dissolved solids (TDS). High-quality deionized water has a conductivity of : \kappa = 0.05501 \pm 0.0001 μS/cm at 25 °C. This corresponds to a specific resistivity of : \rho = 18.18 \pm 0.03 MΩ⋅cm. The preparation of salt solutions often takes place in unsealed beakers. In this case the conductivity of purified water often is 10 to 20 times higher. A discussion can be found below. Typical drinking water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trouton's Rule
In thermodynamics, Trouton's rule states that the (molar) entropy of vaporization has almost the same value, about 85–88 J/(K·mol), for various kinds of liquids at their boiling points. The entropy of vaporization is defined as the ratio between the enthalpy of vaporization and the boiling temperature. It is named after Frederick Thomas Trouton. It is expressed as a function of the gas constant : : \Delta \bar S_\text \approx 10.5 R. A similar way of stating this (Trouton's ratio) is that the latent heat is connected to boiling point roughly as : \frac \approx 8588\ \frac. Trouton’s rule can be explained by using Boltzmann's definition of entropy to the relative change in free volume (that is, space available for movement) between the liquid and vapour phases. It is valid for many liquids; for instance, the entropy of vaporization of toluene is 87.30 J/(K·mol), that of benzene is 89.45 J/(K·mol), and that of chloroform is 87.92 J/(K·mol). Because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |