|
Sienite
Syenite is a coarse-grained intrusive igneous rock with a general composition similar to that of granite, but deficient in quartz, which, if present at all, occurs in relatively small concentrations (< 5%). Some syenites contain larger proportions of components and smaller amounts of felsic material than most granites; those are classed as being of . The equivalent of syenite is |
Syenite
Syenite is a coarse-grained intrusive igneous rock with a general composition similar to that of granite, but deficient in quartz, which, if present at all, occurs in relatively small concentrations (< 5%). Some syenites contain larger proportions of mafic components and smaller amounts of material than most granites; those are classed as being of intermediate composition. The extrusive equivalent of syenite is trachyte. Composition of syenites The[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
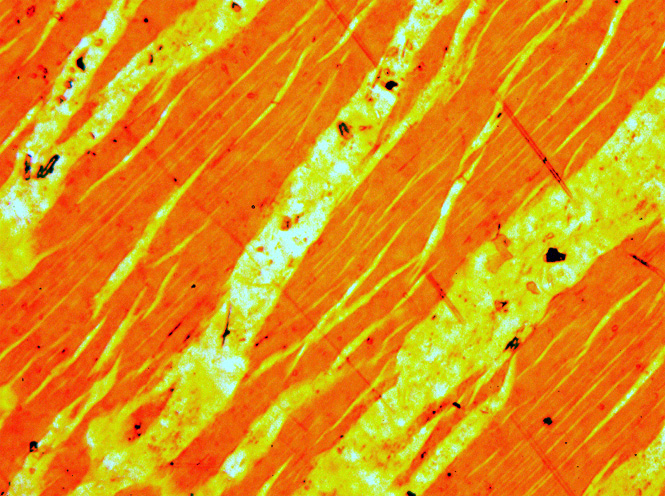
Perthite
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains sepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and read ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peraluminous
Peraluminous rocks are igneous rocks that have a molecular proportion of aluminium oxide higher than the combination of sodium oxide, potassium oxide and calcium oxide.Blatt, Harvey and Robert J. Tracy, ''Petrology,'' Freeman, 2nd ed., 1995, p. 516 This contrasts with peralkaline in which the alkalis are higher, metaluminous where aluminium oxide concentration is lower than the combination, but above the alkalis, and subaluminous in which aluminia concentration is lower than the combination. Examples of peraluminous minerals include biotite, muscovite, cordierite, andalusite and garnet. Peraluminous corresponds to the aluminum saturation index values greater than 1. Peraluminous magmas can form S-type granitoids and have been linked to collisional orogenies and to the formation of tin, tungsten and silver deposits such as those in the Bolivian tin belt The Bolivian tin belt ( es, Cinturón estanifero boliviano, provincia estannifera boliviana) is a mineral-rich region in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peralkaline
Peralkaline rocks include those igneous rocks which have a deficiency of aluminium such that sodium and potassium are in excess of that needed for feldspar. The presence of aegerine (sodium pyroxene) and riebeckite (sodium amphibole) are indicative of peralkaline conditions. Examples are the peralkaline rhyolites, comendite and pantellerite, with comendite being the more felsic (silica-rich) rock. Another example is the peralkaline granite that forms the islet of Rockall in the North Atlantic Ocean. Peralkaline rocks are indicative of continental rift-related volcanicity (e.g. the peralkaline rhyolites of the East African Rift in central Kenya) as well as continental and oceanic hotspot volcanicity (e.g. the peralkaline rhyolites of the Glass House Mountains in eastern Australia and the Canary Islands in the Atlantic Ocean). Peralkaline rocks related to subduction zone volcanicity have also been reported (e.g. Sardinia in Italy). Peralkaline magmas likely form when fractional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green. The name derives from the Persian ''zargun'', meaning "gold-hued". This word is corrupted into " jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from ''Zirkon'', which is the German adaptation of this word. Yellow, orange, and red zircon is also known as " hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanite
Titanite, or sphene (from the Greek ''sphenos'' (σφηνώ), meaning wedge), is a calcium titanium nesosilicate mineral, Ca Ti Si O5. Trace impurities of iron and aluminium are typically present. Also commonly present are rare earth metals including cerium and yttrium; calcium may be partly replaced by thorium. Nomenclature The International Mineralogical Association Commission on New Minerals and Mineral Names (CNMMN) adopted the name titanite and "discredited" the name sphene as of 1982, although commonly papers and books initially identify the mineral using both names. Sphene was the most commonly used name until the IMA decision, although both were well known. Some authorities think it is less confusing as the word is used to describe any chemical or crystal with oxidized titanium such as the rare earth titanate pyrochlores series and many of the minerals with the perovskite structure. The name sphene continues to be publishable in peer-reviewed scientific literatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annite
Annite is a phyllosilicate mineral in the mica family. It has a chemical formula of KFe32+AlSi3O10(OH)2. Annite is the iron end member of the biotite mica group, the iron rich analogue of magnesium rich phlogopite. Annite is monoclinic and contains tabular crystals and cleavage fragments with pseudohexagonal outlines. There are contact twins with composition surface and twin axis . Annite was first described in 1868 for the first noted occurrence in Cape Ann Cape Ann is a rocky peninsula in northeastern Massachusetts, United States on the Atlantic Ocean. It is about northeast of Boston and marks the northern limit of Massachusetts Bay. Cape Ann includes the city of Gloucester and the towns o ..., Rockport, Essex County, Massachusetts, US. It also occurs on Pikes Peak, El Paso County, Colorado. It occurs in igneous and metamorphic rocks that are deficient in magnesium and is associated with fluorite and zircon in the type locality. Properties The reli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phyllosilicates
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is generally a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron- endmember annite, and the magnesium-endmember phlogopite; more aluminous end-members include siderophyllite and eastonite. Biotite was regarded as a mineral ''species'' by the International Mineralogical Association until 1998, when its status was changed to a mineral ''group''. The term ''biotite'' is still used to describe unanalysed dark micas in the field. Biotite was named by J.F.L. Hausmann in 1847 in honor of the French physicist Jean-Baptiste Biot, who performed early research into the many optical properties of mica. Members of the biotite group are sheet silicates. Iron, magnesium, aluminium, silicon, oxygen, and hydrogen form sheets that are weakly bound together by potassium ions. The term "iron mica" is sometimes used for iron-rich biotite, but the term also refers to a flaky mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinopyroxene
The pyroxenes (commonly abbreviated to ''Px'') are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents calcium (Ca), sodium (Na), iron (Fe II) or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron (Fe II) or (Fe III). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes. The name ''pyroxene'' is derived from the Ancient Greek words for 'fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |