|
Polyamine
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Decarboxylase
The enzyme ornithine decarboxylase (, ODC) catalyzes the decarboxylation of ornithine (a product of the urea cycle) to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer. Reaction mechanism Lysine 69 on ornithine decarboxylase (ODC) binds the cofactor pyridoxal phosphate to form a Schiff base. Ornithine displaces the lysine to form a Schiff base attached to orthonine, which decarboxylates to form a quinoid intermediate. This intermediate rearranges to form a Schiff base attached to putrescine, which is attacked by lysine to release putrescine product and reform PLP-bound ODC. This is the first step and the rate-limiting step in humans for the production of polyamines, compounds required for cell division. Structure image:Ornithine Decarboxylase Publication View.png, 270px, 3D crystal structure of ornithine decarboxylase.; ; rendered viPyMOL The active form of ornithine decarboxyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermidine
Spermidine is a polyamine compound () found in ribosomes and living tissues and having various metabolic functions within organisms. It was originally isolated from semen. Function Spermidine is an aliphatic polyamine. Spermidine synthase (SPDS) catalyzes its formation from putrescine. It is a precursor to other polyamines, such as spermine and its structural isomer thermospermine. Spermidine synchronizes an array of biological processes, (such as Ca2+, Na+, K+ -ATPase) thus maintaining membrane potential and controlling intracellular pH and volume. Spermidine regulates biological processes, such as Ca2+ influx by glutamatergic N-methyl-d-aspartate receptor (NMDA receptor), which has been associated with nitric oxide synthase (NOS) and cGMP/PKG pathway activation and a decrease of Na+,K+-ATPase activity in cerebral cortex synaptosomes. Spermidine is a longevity agent in mammals due to various mechanisms of action, which are just beginning to be understood. Autophagy is the mai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermine
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. Antonie van Leeuwenhoek first described crystals of spermine phosphate in human semen in 1678. The name ''spermin'' was first used by the German chemists Ladenburg and Abel in 1888, and the correct structure of spermine was not finally established until 1926, simultaneously in England (by Dudley, Rosenheim, and Starling) and Germany (by Wrede et al.). Spermine is the chemical primarily responsible for the characteristic odor of semen. Derivative A derivative of spermine, N1, N12-bis(ethyl)spermine (also known as BESm) was investigated in the late 1980s along with similar polyamine analo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Putrescine
Putrescine is an organic compound with the formula (CH2)4(NH2)2. It is a colorless solid that melts near room temperature. It is classified as a diamine. Together with cadaverine, it is largely responsible for the foul odor of putrefying flesh, but also contributes to other unpleasant odors. Production Putrescine is produced on an industrial scale by the hydrogenation of succinonitrile. Biotechnological production of putrescine from renewable feedstock has been investigated. A metabolically engineered strain of ''Escherichia coli'' that produces putrescine at high concentrations in glucose mineral salts medium has been described. Biochemistry Spermidine synthase uses putrescine and ''S''-adenosylmethioninamine (decarboxylated ''S''-adenosyl methionine) to produce spermidine. Spermidine in turn is combined with another ''S''-adenosylmethioninamine and gets converted to spermine. Putrescine is synthesized in small quantities by healthy living cells by the action of ornit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Tris(aminomethyl)ethane
1,1,1-Tris(aminomethyl)ethane (TAME) is an organic compound with the formula CHC(CHNH). It is a colorless liquid. It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron. Preparation TAME is synthesized by the Pd/C-catalyzed hydrogenation of 1,1,1-tris(azidomethyl)ethane. Although azides are potentially explosive, they are excellent and practical source of primary amines. The required tris(azidomethyl)ethane is obtained from the tritosylate by salt metathesis using sodium azide. These two steps are: :3 NaN + CHC(CHOTs) → CHC(CHN) + 3 NaOTs :3 H + CHC(CHN) → CHC(CHNH) + 3 N Complexes of TAME The tripodal TAME ligand In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy
Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (usually called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of applications, including metal coatings, composites, use in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylenetetramine
Triethylenetetramine (TETA and trien), also known as trientine (INN) when used medically, is an organic compound with the formula H2NHCH2CH2NH2sub>2. The pure freebase is a colorless oily liquid, but, like many amines, older samples assume a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA. The hydrochloride salts are used medically as a treatment for copper toxicity. Uses The reactivity and uses of TETA are similar to those for the related polyamines ethylenediamine and diethylenetriamine. It is primarily used as a crosslinker ("hardener") in epoxy curing. Medical uses The hydrochloride salt of TETA, referred to as ''trientine hydrochloride'', is a chelating agent that is used to bind and remove copper in the body to treat Wilson's disease, particularly in those who are intolerant to penicillamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for o(tren)X2sup>+, where X is halide or pseudohalide. In contrast, for o(trien)X2sup>+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common triethylenetet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living thin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
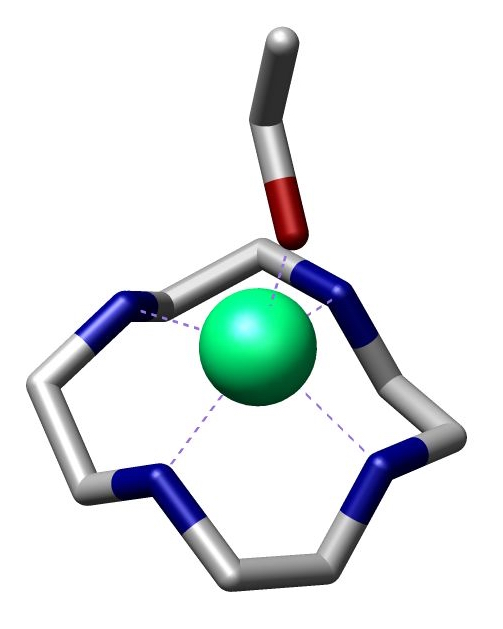
Cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is a aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. Synthesis Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates: :TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4 The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine. High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL. : In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine. The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N–H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes. ''N''-Alkyl derivatives Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aziridine
Aziridine is an organic compound consisting of the three-membered heterocycle . It is a colorless, toxic, volatile liquid that is of significant practical interest. Aziridine was discovered in 1888 by the chemist Siegmund Gabriel. Its derivatives, also referred to as aziridines, are of broader interest in medicinal chemistry. Structure The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrogen free electron pair. Angle strain in aziridine also increases the barrier to nitrogen inversion. This barrier height permits the isolation of separate ''invertomers'', for example the ''cis'' and ''trans'' invertomers of ''N''-chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

Cl2.png)