|
Proglumetacin
Proglumetacin (usually as the maleate salt, trade names Afloxan, Protaxon and Proxil) is a nonsteroidal anti-inflammatory drug (NSAID). It is metabolized in the body to indometacin and proglumide Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist, which blocks both the CCKA and CCKB subtypes. It was used mainly in the treatment of stomach ulcer ..., a drug with antisecretory effects that helps prevent injury to the stomach lining. References Nonsteroidal anti-inflammatory drugs Prodrugs Indole ethers at the benzene ring Piperazines Carboxylate esters Benzamides {{musculoskeletal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proglumide
Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist, which blocks both the CCKA and CCKB subtypes. It was used mainly in the treatment of stomach ulcers, although it has now been largely replaced by newer drugs for this application. An interesting side effect of proglumide is that it enhances the analgesia produced by opioid drugs, and can prevent or even reverse the development of tolerance Tolerance or toleration is the state of tolerating, or putting up with, conditionally. Economics, business, and politics * Toleration Party, a historic political party active in Connecticut * Tolerant Systems, the former name of Veritas Software ... to opioid drugs. This can make it a useful adjuvant treatment to use alongside opioid drugs in the treatment of chronic pain conditions such as cancer, where opioid analgesics may be required for long periods and development of tolerance reduces cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it is located in the right upper quadrant of the abdomen, below the diaphragm. Its other roles in metabolism include the regulation of glycogen storage, decomposition of red blood cells, and the production of hormones. The liver is an accessory digestive organ that produces bile, an alkaline fluid containing cholesterol and bile acids, which helps the breakdown of fat. The gallbladder, a small pouch that sits just under the liver, stores bile produced by the liver which is later moved to the small intestine to complete digestion. The liver's highly specialized tissue, consisting mostly of hepatocytes, regulates a wide variety of high-volume biochemical reactions, including the synthesis and breakdown of small and complex molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterohepatic Circulation
Enterohepatic circulation refers to the circulation of biliary acids, bilirubin, drugs or other substances from the liver to the bile, followed by entry into the small intestine, absorption by the enterocyte and transport back to the liver. Enterohepatic circulation is an especially important concept in the field of toxicology as many lipophilic xenobiotics undergo this process causing repeated liver damage. Biliary acids The circuit Hepatocytes metabolize cholesterol to cholic acid and chenodeoxycholic acid. These lipid-soluble bile acids are conjugated (reversibly attached) mainly to glycine or taurine molecules to form water soluble primary conjugated bile acids, sometimes called "bile salts". These bile acids travel to the gall bladder during the interdigestive phase for storage and to the descending part of the duodenum via the common bile duct through the major duodenal papilla during digestion. 95% of the bile acids which are delivered to the duodenum will be recycled b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maleate
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric acid is the ''trans''-isomer. It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications. Physical properties Maleic acid has a ''heat of combustion'' of -1,355 kJ/mol., 22.7 kJ/mol higher than that of fumaric acid. Maleic acid is more soluble in water than fumaric acid. The melting point of maleic acid (135 °C) is also much lower than that of fumaric acid (287 °C). Both properties of maleic acid can be explained on account of the intramolecular hydrogen bonding that takes place in maleic acid at the expense of intermolecular interactions, and that are not possible in fumaric acid for geometric reasons. Production and industrial applications In industry, male ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
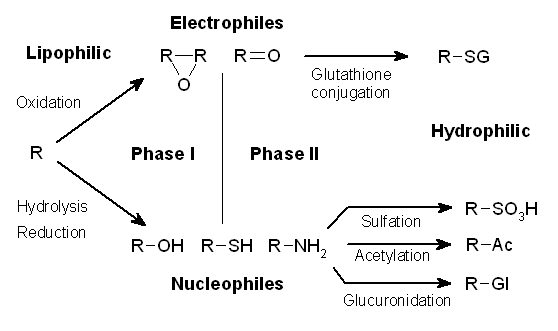
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is called pharmacokinetics. The metabolism of pharmaceutical drugs is an important aspect of pharmacology and medicine. For example, the rate of metabolism determines the duration and intensity of a drug's pharmacologic action. Drug metabolism also affects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indometacin
Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription medication to reduce fever, pain, stiffness, and swelling from inflammation. It works by inhibiting the production of prostaglandins, endogenous signaling molecules known to cause these symptoms. It does this by inhibiting cyclooxygenase, an enzyme that catalyzes the production of prostaglandins. It was patented in 1961 and approved for medical use in 1963. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 320th most commonly prescribed medication in the United States, with more than 800thousand prescriptions. Medical uses As an NSAID, indometacin is an analgesic, anti-inflammatory, and antipyretic. Clinical indications for indometacin include: Joint diseases *rheumatoid arthritis *ankylosing spondylitis *osteoarthritis *gouty arthritis *acute painful shoulder bursitis or tendinitis Headaches *Trigeminal autonomic ceph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prodrugs
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug is absorbed, distributed, metabolized, and excreted (ADME). Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract. A prodrug may be used to improve how selectively the drug interacts with cells or processes that are not its intended target. This reduces adverse or unintended effects of a drug, especially important in treatments like chemotherapy, which can have severe unintended and undesirable side effects. History Many herbal extracts historically used in medicine contain glycosides (sugar derivatives) of the active agent, which are hydrolyzed in the intestines to release the active and more bioavailable aglycone. For example, salicin is a β-D-glucopyran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole Ethers At The Benzene Ring
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperazines
Substituted piperazines are a class of chemical compounds based on a piperazine core. Some are used as recreational drugs and some are used in scientific research. List of substituted piperazines Benzylpiperazines File:Benzylpiperazine.svg, 1-Benzylpiperazine File:MBZP.svg, 1-Methyl-4-benzylpiperazine File:DBZP.svg, 1,4-Dibenzylpiperazine File:MDBZP.svg, 3,4-Methylenedioxy-1-benzylpiperazine File:2C-B-BZP.svg, 4-Bromo-2,5-dimethoxy-1-benzylpiperazine File:Methoxypiperamide.png, Methoxypiperamide File:Sunifiram.svg , Sunifiram File:3-Methylbenzylpiperazine structure.png, 3-Methylbenzylpiperazine * 1-Benzylpiperazine (BZP) * 1-Methyl-4-benzylpiperazine (MBZP) * 1,4-Dibenzylpiperazine (DBZP) * 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP) * 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) * Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone) * Sunifiram (1-benzoyl-4-propanoylpiperazine) * 3-Methylbenzylpiperazine (3-MeBZP) Befuralin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |