|
Nonsteroidal Anti-inflammatory Drugs
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents. List of nonsteroidal steroid receptor modulators Examples include the following: * Estrogens: benzestrol, bifluranol, estrobin, estrobin (DBE), diethylstilbestrol, diethylstilbestrol (stilbestrol), dienestrol, erteberel, fosfestrol, hexestrol, hexestrol (dihydroxystilbestrol), methallenestril, methestrol, methestrol dipropionate, paroxypropione, prinaberel, and triphenylethylene, as well as many xenoestrogens * : acolbifene, afimoxifene, arzoxifene, bazedoxifene, broparestrol, chlorotrianisene, clomifene, clomifenoxide, cyclofenil, droloxifene, enclomifene, endoxifen, ethamoxytriphetol, fispemifene, idoxifene, lasofoxifene, levormeloxifene, miproxifene, nafoxidine, nitromifene, ormeloxifene, ospemifene, panomifene, pipendoxifene, raloxifene, tamoxifen, toremifene, trioxifene, zind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
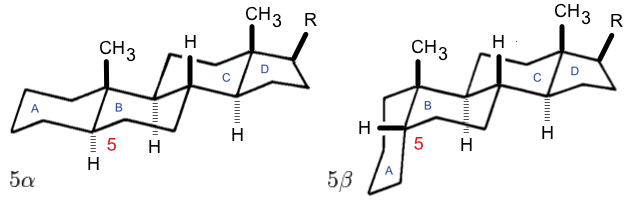
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prinaberel
Prinaberel (International Nonproprietary Name, INN, United States Adopted Name, USAN) (developmental code names ERB-041, WAY-202041) is a synthetic compound, synthetic, nonsteroidal, and highly binding selectivity, selective agonist of the estrogen receptor beta, ERβ isoform, subtype of the estrogen receptor. It is used in scientific research to elucidate the role of the ERβ receptor. Studies have indicated that selective ERβ agonists like prinaberel could be useful in the clinical treatment of a variety of medical conditions including inflammatory bowel disease, rheumatoid arthritis, endometriosis, and sepsis. Accordingly, prinaberel either was or still is under investigation by Wyeth for the treatment of some of these conditions. See also * Diarylpropionitrile * ERB-196 * Erteberel * WAY-200070 * WAY-214156 References External links Prinaberel - AdisInsight Benzoxazoles Fluoroarenes Selective ERβ agonists {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enclomifene
Enclomifene (), or enclomiphene (), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group, acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (''E'')- stereoisomer of clomifene, while zuclomifene is the (''Z'')-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism. Enclomiphene (former tentative brand names Androxal and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Droloxifene
Droloxifene (International Nonproprietary Name, INN, United States Adopted Name, USAN) (former developmental code names FK-435, ICI-79280, K-060, K-21060E, RP-60850), also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and menopause, postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached Phases of clinical research, phase II and Phases of clinical research#Phase III, phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials. Droloxifene is an structural analogue, analogue of tamoxifen, specifically 3-hydroxytamoxifen, but has been said to have 10- to 60-fold increased affinity (ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclofenil
Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women. It is mostly no longer available. The medication is taken by mouth. Side effects of cyclofenil include liver toxicity among others. It is a selective estrogen receptor modulator (SERM) and hence is a mixed agonist–antagonist of the estrogen receptor (ER), the biological target of estrogens like estradiol. It has antiestrogenic effects on the hypothalamic–pituitary–gonadal axis and hence can increase sex hormone production and stimulate ovulation. Cyclofenil was introduced for medical use in 1970. It has been mostly discontinued, but remains available in a few countries, including Brazil, Italy, and Japan. It has been used as a doping agent by male athletes. Medical use Cyclofenil is used to treat menstrual disturbances and anovulatory inferti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clomifenoxide
Clomifenoxide (International Nonproprietary Name, INN), also known as clomifene ''N''-oxide, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that is described as an antiestrogen and "progonadotropin, gonad stimulant" and was never marketed. It is an active metabolite of clomifene. See also * Afimoxifene * Endoxifen References Human drug metabolites Organochlorides Progonadotropins Selective estrogen receptor modulators Triphenylethylenes Amine oxides {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clomifene
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth. Common side effects include pelvic pain and hot flashes. Other side effects can include changes in vision, vomiting, trouble sleeping, ovarian cancer, and seizures. It is not recommended in people with liver disease or abnormal vaginal bleeding of unknown cause or who are pregnant. Clomifene is in the selective estrogen receptor modulator (SERM) family of medication and is a nonsteroidal medication. It works by causing the release of GnRH by the hypothalamus, and subsequently gonadotropin from the anterior pituitary. Clomifene was approved for medical use in the United States in 1967. It is on the World Health Organization's List of Essential Medicines. Its introduction began the era of assisted reproductive technology. Clomifene (particularly the purified enclomiphene isomer) has also been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrianisene
Chlorotrianisene (CTA), also known as tri-''p''-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth. CTA is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol. It is a high-efficacy partial estrogen and shows some properties of a selective estrogen receptor modulator, with predominantly estrogenic activity but also some antiestrogenic activity. CTA itself is inactive and is a prodrug in the body. CTA was introduced for medical use in 1952. It has been marketed in the United States and Europe. However, it has since been discontinued and is no longer available in any country. Medical uses CTA has been u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Broparestrol
Broparestrol () (brand names Acnestrol, Longestrol; former developmental code name LN-107), also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of ''E-'' and ''Z-'' isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...s (LN-1643 and LN-2299, respectively), both of which are active, and are similarly antiestrogenic b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bazedoxifene
Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly (pending more study) for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention (not treatment) of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer. Medical uses Bazedoxifene is used in the prevention of postmenopausal osteoporosis. Osteoporosis represents a major public health concern, especially as the number of postmenopausal women continues to rise. As a result, the need for innovative treatments has become increasingly important. Bazedoxifene (BZA) has emerged as a promising option for postmenopausal osteoporosis due to its demonstrated effectiveness in reducing bone loss and fractures, as well as its strong safety and tolerability profile. For women who cannot or prefer not to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arzoxifene
Arzoxifene (; developmental code name LY-353381) is a selective estrogen receptor modulator (SERM) of the benzothiophene group which was never marketed. It is a potent estrogen antagonist in mammary and uterine tissue while acting as an estrogen agonist to maintain bone density and lower serum cholesterol. Arzoxifene is a highly effective agent for prevention of mammary cancer induced in the rat by the carcinogen nitrosomethylurea and is significantly more potent than raloxifene in this regard. Arzoxifene is devoid of the uterotrophic effects of tamoxifen, suggesting that, in contrast to tamoxifen, it is unlikely that the clinical use of arzoxifene will increase the risk of developing endometrial carcinoma. Pharmacology Arzoxifene is a selective estrogen receptor modulator (SERM), and hence is a mixed agonist and antagonist of the estrogen receptor with tissue-selective estrogenic and antiestrogenic activity. It has antiestrogenic effects in the breast, mixed estrogenic and anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Afimoxifene
Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed. Afimoxifene is a SERM and hence acts as a tissue-selective agonist–antagonist of the estrogen receptors ERα and ERβ with mixed estrogenic and antiestrogenic activity depending on the tissue. It is also an agonist of the G protein-coupled estrogen receptor (GPER) with relatively low affinity (100–1,000 nM, relative to 3–6 nM for estradiol). In addition to its estrogenic and antiestrogenic activity, afimoxifene has been found to act as an antagoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |