|
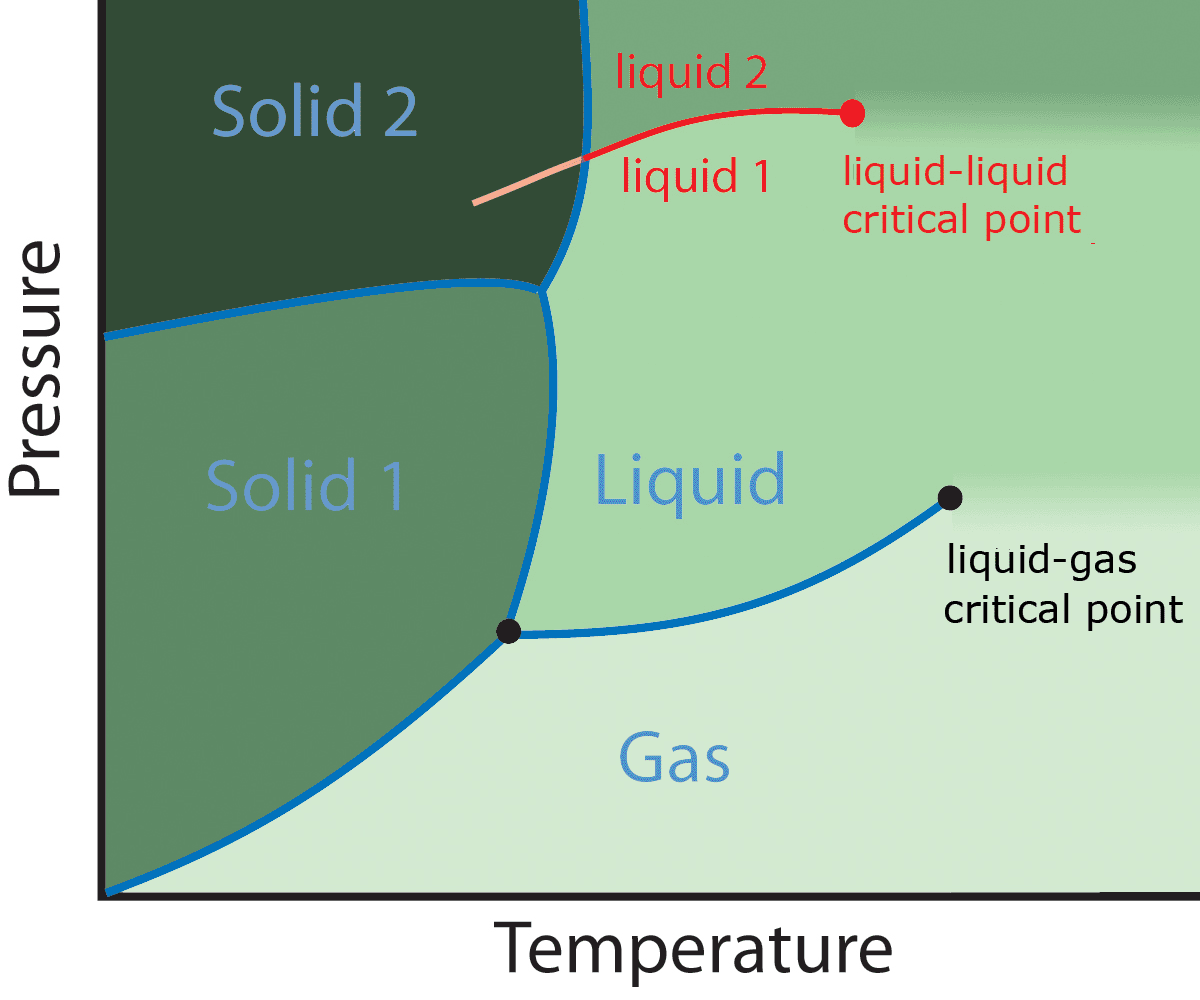
Polyamorphism
Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics (e.g. polymers). However, polyamorphism requires ''two distinct'' amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition. Overview Even though amorphous materials exhibit no long-range periodic atomic ordering, there is still significant and varied local structure at inter-atomic length scales (see structure of liquids and glasses). Different local structures can produce amorphous phases of the same chemical composition with different physical properties such as density. In several cases sharp transitions have been observed between two different density amorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Structure Of Liquids And Glasses
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials. Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions. Metallic glasses, for example, are typically well described by the dense random packing of hard spheres, whereas covalent systems, such as silicate glasses, have sparsely packed, strongly bound, tetrahedral network structures. These very different structures result in materials with very different physical properties and applications. The study of liquid and glass structure aims to gain insight into their behavior and physical properties, so that they can be understood, predicted and tailored for specific applications. Since the structure and resulting behavior of liquids and glasses is a complex many body problem, historically it has been too computation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triphenyl Phosphite
Triphenyl phosphite is the organophosphorus compound with the formula P(OC6H5)3. It is a colourless viscous liquid. Preparation Triphenylphosphite is prepared from phosphorus trichloride and phenol in the presence of a catalytic amount of base: :PCl3 + 3 HOC6H5 → P(OC6H5)3 + 3 HCl Reactions Triphenylphosphite is a precursor to trimethylphosphine, it serves as a source of P3+ that is less electrophilic than phosphorus trichloride: : (C6H5O)3P + 3CH3MgBr → P(CH3)3 + 3"MgBrOC6H5" Triphenylphosphite is quaternized by methyl iodide: : (C6H5O)3P + CH3I → H3(C6H5O)3Psup>+I− Coordination complexes Triphenylphosphite is a common ligand in coordination chemistry. It forms zero-valent complexes of the type M (OC6H5)3sub>4 (M = Ni, Pd, Pt). The nickel complex can be prepared by displacement of the diene from bis(cyclooctadiene)nickel: : Ni(COD)2 + 4 P(OC6H5)3 → Ni (OC6H5)3sub>4 + 2 COD Related complexes are homogeneous catalysts for the hydrocyanation of alkenes. It also form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Liquid Nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose viscosity is about one-tenth that of acetone (i.e. roughly one-thirtieth that of water at room temperature). Liquid nitrogen is widely used as a coolant. Physical properties The diatomic character of the N2 molecule is retained after liquefaction. The weak van der Waals interaction between the N2 molecules results in little interatomic attraction. This is the cause of nitrogen's unusually low boiling point. The temperature of liquid nitrogen can readily be reduced to its freezing point by placing it in a vacuum chamber pumped by a vacuum pump. Liquid nitrogen's efficiency as a coolant is limited by the fact that it boils immediately on contact with a warmer object, enveloping the object in an insulating layer of nitrogen gas bubbles. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in a liquid vibrate more intensely, causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glass Transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. International Organization for Standardization, ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting point, melting temperature, ''T''m, of the cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
N-Butanol
1-Butanol, also known as butan-1-ol or ''n''-butanol, is a Alcohol (chemistry), primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol, ''tert''-butanol. The unmodified term butanol usually refers to the straight chain isomer. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and drinks... It is also a permitted artificial flavorant in the United States, used in butter, cream, fruit, rum, whiskey, ice cream and ices, candy, baked goods, and cordials. It is also used in a wide range of consumer products. The largest use of 1-butanol is as an industrial intermediate, particularly for the manufacture of butyl acetate (itself an artificial flavorant and industrial solvent). It is a petrochemical derived from propylene. Estimated production figures for 1997 are: United States 784,000 tonnes; Western Europe 575,000&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Germanium Dioxide
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula Ge O2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen. Structure The two predominant polymorphs of GeO2 are hexagonal and tetragonal. Hexagonal GeO2 has the same structure as α-quartz, with germanium having coordination number 4. Tetragonal GeO2 (the mineral argutite) has the rutile-like structure seen in stishovite. In this motif, germanium has the coordination number 6. An amorphous (glassy) form of GeO2 is similar to fused silica. Germanium dioxide can be prepared in both crystalline and amorphous forms. At ambient pressure the amorphous structure is formed by a network of GeO4 tetrahedra. At elevated pressure up to approximately 9 GPa the germanium average coordination number steadily increases from 4 to around 5 with a corresponding increase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colours to trace impurities. Rubies are given their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |