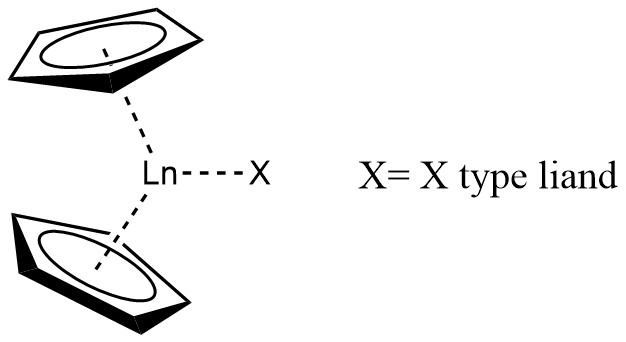|
Organoytterbium Compound
Organolanthanide chemistry is the field of chemistry that studies organolanthanides, compounds with a lanthanide-carbon bond. Organolanthanide compounds are different from their organotransition metal analogues in the following ways: * They are far more air- and water-sensitive and are often pyrophoric. * Chemistry in the 0 oxidation state is far more limited. In fact, their electropositive nature makes their organometallic compounds more likely to be ionic. * They form no stable carbonyls at room temperature; organolanthanide carbonyl compounds have been observed only in argon matrices, and decompose when heated to 40 K. σ-Bonded complexes Metal-carbon σ bonds are found in alkyls of the lanthanide elements such as nMe6sup>3− and Ln H(SiMe3)2sub>3. Methyllithium dissolved in THF reacts in stoichiometric ratio with LnCl3 (Ln = Y, La) to yield Ln(CH3)3 probably contaminated with LiCl. If a chelating agent (L-L), such as tetramethylethylenediamine (tmed or tmeda) or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylmercury
Diphenylmercury is the organomercury compound with the formula . It is a white solid. The compound is of historic interest as a particularly stable organometallic compound but it finds few uses because of its high toxicity. Preparation Commercially available, this compound can be prepared by several routes. It results from treating phenylmercury acetate with sodium stannite, by the reaction of mercuric halides with phenylmagnesium bromide Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It forms colorless crystals. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide i ..., and the reaction of bromobenzene with sodium amalgam. Safety Diphenylmercury is highly toxic. References {{Mercury compounds Organomercury compounds Phenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroalkoxylation
Hydroalkoxylation is a chemical reaction that combines alcohols with alkenes or alkynes. The process affords ethers. The reaction converts alkenes to dialkyl or aryl-alkyl ethers: :R'OH + RCH=CH2 → R'OCH(R)-CH3 Similarly, alkynes are converted to vinyl ethers: :R'OH + RC≡CH → R'OC(R)=CH2 As shown, the reaction follows the Markovnikov rule. The process exhibits good atom-economy in the sense that no byproducts are produced. The reaction is catalyzed by bases and also by transition metal complexes. Usually symmetrical ethers are prepared by dehydration of alcohols and unsymmetrical ethers by the Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ... from alkyl halides and alkali metal alkoxides.Wilhelm Heitmann, Günther Strehlke, Dieter Mayer "Ethers, Ali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroamination
In organic chemistry, hydroamination is the formal Addition reaction, addition of an bond of an amine across an Carbon–carbon bond, carbon-carbon multiple bond of an alkene, alkyne, diene, or allene. In the ideal case, hydroamination is Atom economy, atom economical and Green chemistry, green; and the products could see extensive use in fine-chemical, pharmaceutical, and agricultural industries. Hydroamination reactions occur spontaneously only for electrophilic alkenes and some dienes, but these are known by other names (e.g. Michael addition reaction); "hydroamination" is generally reserved for situations where the reaction requires a catalyst. Hydroamination is however of little value industrially. Hydroamination can be used Intramolecular reaction, intramolecularly to create Heterocyclic compounds, heterocycles or intermolecularly with a separate amine and unsaturated compound. File:Examples of intermolecular hydroamination.png, alt=Prototypical intermolecular hydroaminatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxophilicity
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilicity is often stated to be related to the hardness of the element, within the HSAB theory ( hard and soft (Lewis) acids and bases), but it has been shown that oxophilicity depends more on the electronegativity and effective nuclear charge of the element than on its hardness. This explains why the early transition metals, whose electronegativities and effective nuclear charges are low, are very oxophilic. Many main group compounds are also oxophilic, such as derivatives of aluminium, silicon, and phosphorus(III). The handling of oxophilic compounds often requires air-free techniques. Examples Complexes of oxophilic metals typically are prone to hydrolysis. For example, the high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolanthanide As Electroluminescent Material
Organolanthanide chemistry is the field of chemistry that studies organolanthanides, compounds with a lanthanide-carbon bond. Organolanthanide compounds are different from their organotransition metal analogues in the following ways: * They are far more air- and water-sensitive and are often pyrophoric. * Chemistry in the 0 oxidation state is far more limited. In fact, their electropositive nature makes their organometallic compounds more likely to be ionic. * They form no stable carbonyls at room temperature; organolanthanide carbonyl compounds have been observed only in argon matrices, and decompose when heated to 40 K. σ-Bonded complexes Metal-carbon σ bonds are found in alkyls of the lanthanide elements such as nMe6sup>3− and Ln H(SiMe3)2sub>3. Methyllithium dissolved in THF reacts in stoichiometric ratio with LnCl3 (Ln = Y, La) to yield Ln(CH3)3 probably contaminated with LiCl. If a chelating agent (L-L), such as tetramethylethylenediamine (tmed or tmeda) or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single-molecule Magnet
A single-molecule magnet (SMM) is a metal-organic compound that has superparamagnetic behavior below a certain blocking temperature at the molecular scale. In this temperature range, an SMM exhibits magnetic hysteresis of purely molecular origin.Introduction to Molecular Magnetism by Dr. Joris van Slageren. In contrast to conventional bulk s and molecule-based magnets, collective long-range magnetic ordering of s is not necessary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that Sublimation (phase transition), sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation . Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and respectively. The first reported synthesis of ferrocene was in 1951. Its unusual stability puzzled chemists, and required the development of new theory to explain its formation and bonding. The discovery of ferrocene and its many Structural analog, analogues, known as metallocenes, sparke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanocene
A lanthanocene is a type of metallocene compound that contains an Chemical element, element from the lanthanide series. The most common lanthanocene complexes contain two Sodium cyclopentadienide, cyclopentadienyl anions and an Covalent bond classification method, X type ligand, usually hydride or Allyl ligand, alkyl ligand. History In 1954, Geoffrey Wilkinson, Wilkinson and Birmingham described the tris(cyclopentadienyl)lanthanide complex, Ln(C5H5)3 (Ln = Lanthanum, La, Cerium, Ce, Praseodymium, Pr, Neodymium, Nd, Samarium, Sm). However, due to the highly moisture and oxygen sensitive character of organolanthanide compounds, as well as the incapability of the separation of simple alkyl and aryl derivatives of the type LnR3, this area of organometallic chemistry experienced a period of relative stagnation for two decades before lanthanocene complexes were prepared for some of the later lanthanides (Ln = Gadolinium, Gd, Erbium, Er, Ytterbium, Yb, Lutetium, Lu). Synthesis The syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Examples ''Bis''cyclopentadienyl complexes are called metallocenes. A famous example of this type of complex is ferrocene (FeCp2), which has many analogues for other metals, such as chromocene (CrCp2), cobaltocene (CoCp2), and nickelocene (NiCp2). When the Cp rings are mutually parallel the compound is known as a sandwich complex. This area of organometallic chemistry was first developed in the 1950s. Bent metallocenes are represented by compounds of the type Cp2Lx Some are catalysts for ethylene polymerization. Metallocenes are often thermally stable, and find use as ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals. Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these researchers found lutetium as an impurity in ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain, as he had published his results earlier. He chose the name ''lutecium'' for the new element, but in 1949 the spelling was changed to ''lutetium''. In 1909, the priority was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |