|
Lingual Lipase
Lingual lipase is a member of a family of digestive enzymes called triacylglycerol lipases, EC 3.1.1.3, that use the catalytic triad of aspartate, histidine, and serine to hydrolyze medium and long-chain triglycerides into partial glycerides and free fatty acids. The enzyme, released into the mouth along with the saliva, catalyzes the first reaction in the digestion of dietary lipid, with diglycerides being the primary reaction product. However, due to the unique characteristics of lingual lipase, including a pH optimum 4.5–5.4 and its ability to catalyze reactions without bile salts, the lipolytic activity continues through to the stomach. Enzyme release is signaled by the autonomic nervous system after ingestion, at which time the serous glands under the circumvallate and foliate papillae on the surface of the tongue secrete lingual lipase into the grooves of the papillae, co-localized with fat taste receptors. The hydrolysis of the dietary fats is essential for fat absorpt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acid residues that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, aminoacylase, acylases, lipases and β-lactamases). An acid-base (chemistry), base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the Substrate (chemistry), substrate, forming a covalent intermediate which is then hydrolysed to release the Product (chemistry), product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine, but occasionally threonine or even selenocysteine. The Protein tertiary structure, 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence (Protein primary structure, primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pancreatic Lipase
Pancreatic lipases () are a protein family, family of lipolytic enzymes that hydrolyse ester linkages of triglycerides. Lipases are widely distributed in animals, plants and prokaryotes. At least three tissue-specific isozymes exist in higher vertebrates, pancreatic, hepatic and gastric/lingual. These lipases are closely related to each other and to lipoprotein lipase (), which hydrolyses triglycerides of chylomicrons and very low density lipoproteins (VLDL). Pancreatic lipase contains two protein domains. The one toward the N terminus is an α/β hydrolase, whereas the one toward the C terminus plays a role in binding to colipase, a protein needed in order for the lipase to become activated. The most conserved region in all these proteins is centred on a serine residue which has been shown to participate, with a histidine and an aspartic acid residue, in a charge relay system. Such a region is also present in lipases of prokaryotic origin and in lecithin-cholesterol acyltrans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exocrine Pancreatic Insufficiency
Exocrine pancreatic insufficiency (EPI) is the inability to properly digest food due to a lack or reduction of digestive enzymes made by the pancreas. EPI can occur in humans and is prevalent in many conditions such as cystic fibrosis, Shwachman–Diamond syndrome, different types of pancreatitis, multiple types of diabetes mellitus (Type 1 and Type 2 diabetes), advanced renal disease, older adults, celiac disease, diarrhea-predominant irritable bowel syndrome (IBS-D), inflammatory bowel disease (IBD), HIV, alcohol-related liver disease, Sjogren syndrome, tobacco use, and use of somatostatin analogues. EPI is caused by a progressive loss of the pancreatic cells that make digestive enzymes. Loss of digestive enzymes leads to maldigestion and malabsorption of nutrients from normal digestive processes. EPI can cause symptoms even before reaching the stages of malnutrition: 'mild' or 'moderate' EPI is when fecal elastase levels are <200 ug/g, whereas 'severe' EPI is con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis
Cystic fibrosis (CF) is a genetic disorder inherited in an autosomal recessive manner that impairs the normal clearance of Sputum, mucus from the lungs, which facilitates the colonization and infection of the lungs by bacteria, notably ''Staphylococcus aureus''. CF is a rare genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. The hallmark feature of CF is the accumulation of thick mucus in different organs. Long-term issues include Shortness of breath, difficulty breathing and coughing up mucus as a result of frequent pneumonia, lung infections. Other signs and symptoms may include Sinusitis, sinus infections, failure to thrive, poor growth, Steatorrhea, fatty stool, Nail clubbing, clubbing of the fingers and toes, and infertility in most males. Different people may have different degrees of symptoms. Cystic fibrosis is inherited in an autosomal recessive manner. It is caused by the presence of mutations in both copies (alleles) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mech2
In science fiction, or mechs are giant robots or machines, typically depicted as piloted, humanoid walking vehicles. The term was first used in Japanese (language), Japanese after shortening the English loanword or , but the meaning in Japanese is more inclusive, and or 'giant robot' is the narrower term. Real mechs vary greatly in size and shape, but are distinguished from vehicles by their biomorphic appearance, and are often much larger than human beings. Different Genre#Subgenre, subgenres exist, with varying connotations of realism. The concept of Super Robot and Real Robot are two such examples found in Japanese anime and manga. Real-world piloted robots or non-robots Robot locomotion, robotic platforms, existing or planned, may also be called "mechs". In Japanese, "mechs" may refer to mobile machinery or vehicles (not including aircraft, cars, motorcycles and HGV) in general, piloted or Mobile robot, otherwise. Characteristics 'Mecha' is an abbreviation, first used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
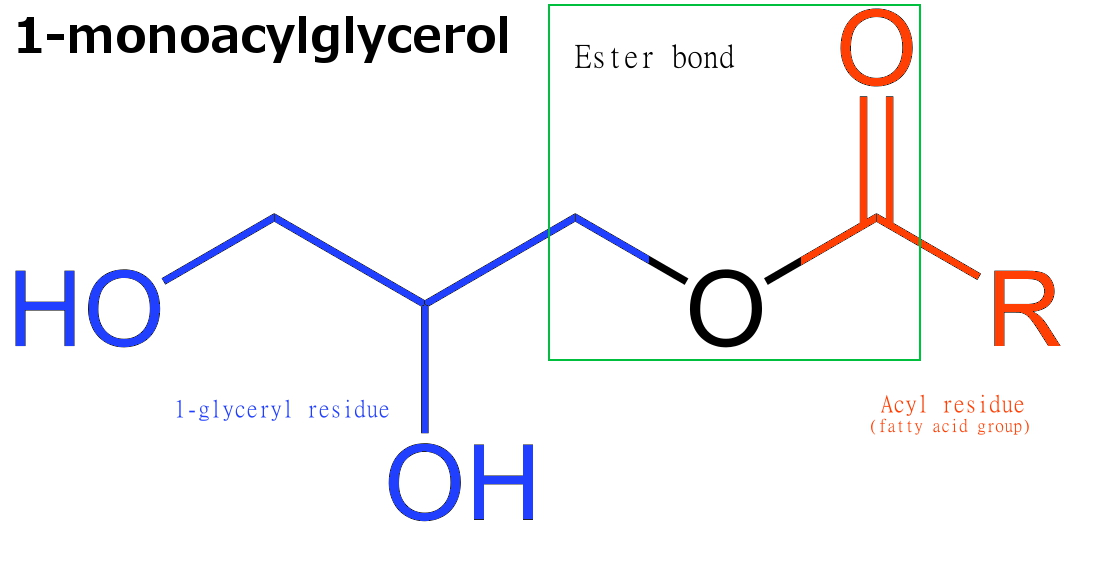
Monoacylglyceride
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol. Synthesis Monoglycerides are produced both biologically and industrially. They are naturally present at very low levels (0.1-0.2%) in some seed oils such as olive oil, rapeseed oil and cottonseed oil. They are biosynthesized by the enzymatic hydrolysis of triglycerides by lipoprotein lipase and the enzymatic hydrolysis of diglycerides by diacylglycerol lipase; or as an intermediate in the alkanoylation of glycerol to form fats. Several monoglycerides are pharmacologically active (e.g. 2-oleoylglycerol, 2-arachidonoylglycerol). Industrial p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triacylglycerol
A triglyceride (from ''tri-'' and '' glyceride''; also TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils. Many types of triglycerides exist. One specific classification focuses on saturated and unsaturated types. Saturated fats have ''no'' C=C groups; unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature. Chemical structure The three fatty acids substituents can be the same, but they are usually different. The positions of the three fatty acids are specified using stereospecific numbering as sn-1, sn-2, and sn-3. The c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pair, Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the Molecular geometry, shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |