|
Hydrogen Difluoride
The bifluoride ion is an inorganic anion with the chemical formula . The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves electrolysis of bifluoride salts. Structure and bonding The bifluoride ion has a linear, centrosymmetric structure (''D∞h'' symmetry), with an F− H bond length of 114 pm. The bond strength is estimated to be greater than 155 kJ/mol. In molecular orbital theory, the atoms are modeled to be held together by a 3-center 4-electron bond ( symmetrical hydrogen bond), in a sort of hybrid between a hydrogen bond and a covalent bond. Reactions Salts, such as potassium bifluoride and ammonium bifluoride Ammonium bifluoride is an inorganic compound with the formula or . It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-industrial etching, etchant and an intermediate in a once-contemplated route to h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
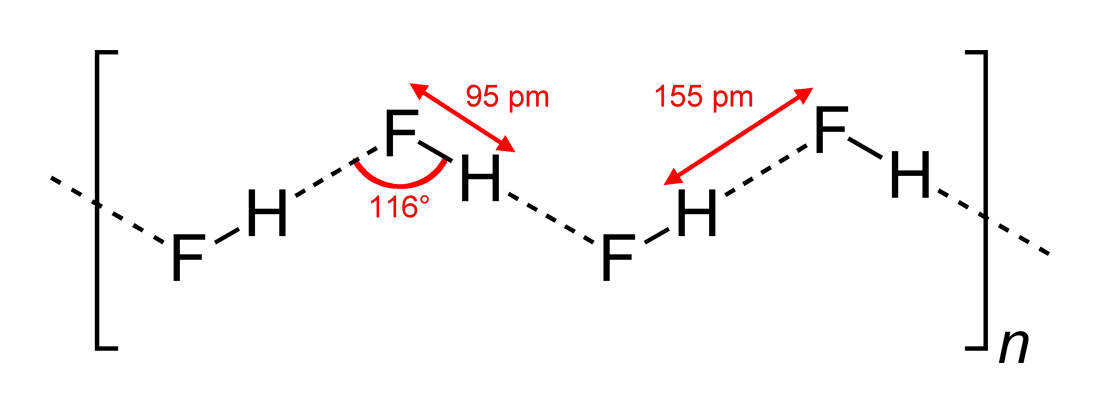
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-center 4-electron Bond
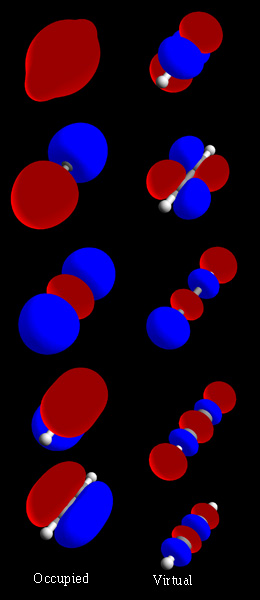
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also known as the Pimentel–Rundle three-center model after the work published by George C. Pimentel in 1951,Pimentel, G. C. The Bonding of Trihalide and Bifluoride Ions by the Molecular Orbital Method. ''J. Chem. Phys.'' 1951, ''19'', 446-448. which built on concepts developed earlier by Robert E. Rundle for electron-deficient bonding.Rundle, R. E. Electron Deficient Compounds. II. Relative Energies of "Half-Bonds". ''J. Chem. Phys.'' 1949, ''17'', 671–675. An extended version of this model is used to describe the whole class of hypervalent molecules such as phosphorus pentafluoride and sulfur hexafluoride as well as multi-center π-bonding such as ozone and sulfur trioxide. There are also molecules such as diborane (B2H6) and dialane (Al2H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etching (microfabrication)
Etching is used in microfabrication to chemically remove layers from the surface of a wafer (electronics), wafer during manufacturing. Etching is a critically important process module in fabrication, and every wafer undergoes many etching steps before it is complete. For many etch steps, part of the wafer is protected from the etchant by a "masking" material which resists etching. In some cases, the masking material is a photoresist which has been patterned using photolithography. Other situations require a more durable mask, such as silicon nitride. Etching media and technology The two fundamental types of etchants are liquid-phase ("wet") and plasma (physics), plasma-phase ("dry"). Each of these exists in several varieties. Wet etching The first etching processes used liquid-phase ("wet") etchants. This process is now largely outdated but was used up until the late 1980s when it was superseded by dry plasma etching. The wafer can be immersed in a bath of etchant, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Bifluoride
Ammonium bifluoride is an inorganic compound with the formula or . It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-industrial etching, etchant and an intermediate in a once-contemplated route to hydrofluoric acid. Structure Ammonium bifluoride, as its name indicates, contains an ammonium cation (), and a bifluoride (or hydrogen difluoride) anion (). The triatomic bifluoride anion features a strong three-center four-electron bond (specifically, a symmetrical hydrogen bond) with a bond energy greater than 155 kJ/mol, and an H-F length of 114 pm. In solid form (), ammonium biflouride is similar to other fluoride salts. Its crystal system is considered orthorhombic, with each cation coordinated with four anions in a tetrahedron (and vice versa). Hydrogen atoms in the ammonium ion form hydrogen bonds with the fluorine atoms, and in the resulting structure, N-H-F are roughly colinear. As a result of these hydrogen bonds, this crystal structure varie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bifluoride
Potassium bifluoride is the inorganic compound with the formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products. Synthesis and reactions The salt was prepared by Edmond Frémy by treating potassium carbonate or potassium hydroxide with hydrofluoric acid: : With one more equivalent of HF, ( CAS RN 12178-06-2, 71.7 °C) is produced: : Thermal decomposition of gives hydrogen fluoride: : Applications The industrial production of fluorine entails the electrolysis of molten and . The electrolysis of was first used by Henri Moissan in 1886. See also * Ammonium bifluoride Ammonium bifluoride is an inorganic compound with the formula or . It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-industrial etching, etchant and an intermediate in a once-contemplated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term "covalence" was introduced by Irving Langmuir in 1919, with Nevil Sidgwick using "co-valent link" in the 1920s. Merriam-Webster dates the specific phrase ''covalent bond'' to 1939, recognizing its first known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetrical Hydrogen Bond
A symmetric hydrogen bond is a special type of hydrogen bond in which the proton is spaced exactly halfway between two identical atoms. The strength of the bond to each of those atoms is equal. It is an example of a 3-center 4-electron bond. This type of bond is much stronger than "normal" hydrogen bonds, in fact, its strength is comparable to a covalent bond. It is seen in ice at high pressure ( Ice X), and also in the solid phase of many anhydrous acids such as hydrofluoric acid and formic acid at high pressure. It is also seen in the bifluoride ion −H−Fsup>−. Much has been done to explain the symmetric hydrogen bond quantum-mechanically, as it seems to violate the duet rule for the first shell: The proton is effectively surrounded by four electrons. Because of this problem, some consider it to be an ionic bond Ionic bonding is a type of chemical bond A chemical bond is the association of atoms or ions to form molecules, crystals, and other structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule by forming a valence chemical bond, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important Reagent, chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |