|
Good Laboratory Practice
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of products in development for human or animal health (including pharmaceuticals) through non-clinical safety tests; from physio-chemical properties through acute to chronic toxicity tests. GLP was first introduced in New Zealand and Denmark in 1972, and later in the US in 1978 in response to the Industrial BioTest Labs scandal. It was followed a few years later by the Organization for Economic Co-operation and Development (OECD) Principles of GLP in 1992; the OECD has since helped promulgate GLP to many countries. GLP applies to non-clinical studies conducted for the assessment of the safety or efficacy of products in development (including pharmaceuticals) for people, animals, and the environment. GLP, a data and oper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratory services are provided in a variety of settings: physicians' offices, clinics, hospitals, and regional and national referral centers. Overview The organisation and contents of laboratories are determined by the differing requirements of the specialists working within. A physics laboratory might contain a particle accelerator or vacuum chamber, while a metallurgy laboratory could have apparatus for casting or refining metals or for testing their strength. A chemist or biologist might use a wet laboratory, while a psychologist's laboratory might be a room with one-way mirrors and hidden cameras in which to observe behavior. In some laboratories, such as those commonly used by computer scientists, computers (sometimes supercomputers) are used for either simulations or the analysis of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OECD Guidelines For The Testing Of Chemicals
OECD Guidelines for the Testing of Chemicals (OECD TG) are a set of internationally accepted specifications for the testing of chemicals decided on by the Organisation for Economic Co-operation and Development (OECD). They were first published in 1981. They are split into five sections: * Section 1: Physical Chemical Properties * Section 2: Effects on Biotic Systems * Section 3: Environmental Fate and Behaviour * Section 4: Health Effects * Section 5: Other Test Guidelines Guidelines are numbered with three digit numbers, the section number being the first number. Sometimes guidelines are suffixed with a letter. Guidelines are under constant review, with guidelines being periodically updated, new guidelines being adopted, and guidelines being withdrawn. Previous guidelines are maintained on the website for reference purposes. Animal welfare concerns are dealt with by ensuring that animal tests are only permitted where necessary. The guidelines are available in both English ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ISO 15189
''ISO 15189 Medical laboratories — Requirements for quality and competence'' is an international standard that specifies the quality management system requirements particular to medical laboratories. The standard was developed by the International Organisation for Standardization's Technical Committee 212 (ISO/TC 212). ISO/TC 212 assigned ISO 15189 to a working group to prepare the standard based on the details of ISO/IEC 17025:1999 ''General requirements for the competence of testing and calibration laboratories''. This working group included provision of advice to medical laboratory users, including specifics on the collection of patient samples, the interpretation of test results, acceptable turnaround times, how testing is to be provided in a medical emergency, and the lab's role in the education and training of health care staff. While the standard is based on ISO/IEC 17025 and ISO 9001, it is a unique document that takes into consideration the specific requirements of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Federation Of Clinical Chemistry And Laboratory Medicine
The International Federation of Clinical Chemistry and Laboratory Medicine or IFCC is a global organization that promotes the fields of clinical chemistry and laboratory medicine. It was established in 1952 as the International Association of Clinical Biochemists to organize the various national societies of these fields. The organization aims to transcend the boundaries of the field of clinical chemistry and laboratory medicine, to build professionalism of members worldwide, to disseminate information on ”best practice” at various levels of technology and of economic development, to provide a forum of standardization and traceability, to enhance the scientific level and the quality of diagnosis and therapy for patients. The IFCC membership comprises 95 national societies and is associated with 6 regional Federations, 55 corporate members and 21 affiliate members representing more than 45,000 laboratory medicine specialists worldwide. Structure and organization The IFCC carr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Laboratory Accreditation Cooperation
The International Laboratory Accreditation Cooperation or ILAC started as a conference in 1977 with the aim of developing international cooperation for facilitating trade by promotion of the acceptance of accredited test and calibration results. In 1996, ILAC became a formal cooperation with a charter to establish a network of mutual recognition agreements among accreditation bodies that would fulfil this aim. The ultimate aim of the ILAC is increased use and acceptance by industry as well as government of the results from accredited laboratories, including results from laboratories in other countries. In this way, the free-trade goal of a 'product tested once and accepted everywhere' can be realised. See also * Accreditation * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * International Federation of Clinical Chemistry and Laboratory Medicine * ISO/IEC 17025 ISO/IEC 17025 General requirements for the competence of testing an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joint Committee For Traceability In Laboratory Medicine
The Joint Committee for Traceability in Laboratory Medicine or JCTLM is collaboration between the International Committee for Weights and Measures (CIPM), the International Federation for Clinical Chemistry and Laboratory Medicine (IFCC), and the International Laboratory Accreditation Cooperation (ILAC). The goal of the JCTLM is to provide a worldwide platform to promote and give guidance on internationally recognized and accepted equivalence of measurements in laboratory medicine and traceability to appropriate measurement standards. See also * Good laboratory practice (GLP) * Institute for Reference Materials and Measurements (IRMM) * Reference range In medicine and health-related fields, a reference range or reference interval is the range or the interval of values that is deemed normal for a physiological measurement in healthy persons (for example, the amount of creatinine in the blood, o ... * Reference values References External links Joint Committee for Trace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Automated Manufacturing Practice
Good automated manufacturing practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. More specifically, the ISPE's guide ''The Good Automated Manufacturing Practice (GAMP) Guide for Validation of Automated Systems in Pharmaceutical Manufacture'' describes a set of principles and procedures that help ensure that pharmaceutical products have the required quality. One of the core principles of GAMP is that quality cannot be tested into a batch of product but must be built into each stage of the manufacturing process. As a result, GAMP covers all aspects of production; from the raw materials, facility and equipment to the training and hygiene of staff. Standard operating procedures (SOPs) are essential for processes that can affect the quality of the finished product. A group of pharmaceutical professionals have banded toget ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Good Clinical Practice
Good clinical practice (GCP) is an international quality standard, which governments can then transpose into regulations for clinical trials involving human subjects. GCP follows the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and enforces tight guidelines on ethical aspects of clinical research. High standards are required in terms of comprehensive documentation for the clinical protocol, record keeping, training, and facilities, including computers and software. Quality assurance and inspections ensure that these standards are achieved. GCP aims to ensure that the studies are scientifically authentic and that the clinical properties of the investigational product are properly documented. GCP guidelines include protection of human rights for the subjects and volunteers in a clinical trial. It also provides assurance of the safety and efficacy of the newly developed compounds. GCP guidelines include standards on ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klimisch Score
The Klimisch score is a method of assessing the reliability of toxicological studies, mainly for regulatory purposes, that was proposed by H.J. Klimisch, M. Andreae and U. Tillmann of the chemical company BASF in 1997 in a paper entitled ''A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data'' which was published in ''Regulatory Toxicology and Pharmacology''. It assigns studies to one of four categories as follows: The applicable guidelines are the (OECD Guidelines for the Testing of Chemicals, EU Test Methods), and other such methods. Often studies are performed to more than one test guideline where they are in agreement as to the requirements. GLP is Good Laboratory Practice. The scoring system is the standard method used in both the EU regulatory schemes (e.g. REACH Regulation). Generally, only Klimisch scores of 1 or 2 can be used by themselves to cover an endpoint. However, Klimisch score 3 and 4 data can still be used a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocidal Products Directive
The Biocidal Products Directive (BPD) also known as the Biocides Directive is European Union Directive, (98/8/EC), which concerns biocides. It is officially known as Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market."Directive 98/8/EC concerning the placing of biocidal products on the market" European Parliament & Council, 16 February 1998. Retrieved 7 April 2015 In 2013 the Biocidal Products Directive was superseded by The Biocidal Products Regulation (BPR, Regulation (EU) 528/2012). Definition Biocide is defined in Article 2(1)(a) as "active substances and preparations containing one or more active substances, put ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
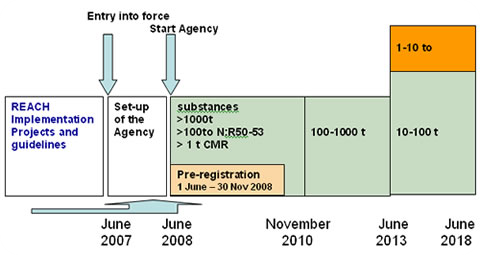
REACH Regulation
Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH. Overview When REACH is fully in force, it will require all companies manufacturing or importing chemical substances into the European Union in quantities of one tonne or more per year to register these substances with a n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |