|
Guanidinoacetic Acid
Glycocyamine (or guanidinoacetate) is a metabolite of glycine in which the amino group has been converted into a guanidine by guanylation (transfer of a guanidine group from arginine). In vertebrate organism it is then transformed into creatine by methylation. Glycocyamine is used as a supplement and as a feed additive in poultry farming. However, the metabolism of creatine from glycocyamine in the liver causes a depletion of methyl groups. This causes homocysteine levels to rise, which has been shown to produce cardiovascular and skeletal problems. Glycocyamine plays a role in the metabolism of the amino acids serine, threonine, and proline. Production Biochemical synthesis Glycocyamine is formed in the mammalian organism primarily in the kidneys by transferring the guanidine group of L-arginine by the enzyme L-Arg:Gly-amidinotransferase (AGAT) to the amino acid glycine. From L-arginine, ornithine is thus produced, which is metabolized in the urea cycle by carbamoylation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcosine
Sarcosine, also known as ''N''-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine. Sarcosine is ubiquitous in biological materials. It is used in manufacturing biodegradable surfactants and toothpastes as well as in other applications. It is also a reagent in organic synthesis. Sarcosine is sweet to the taste. Biochemistry Sarcosine is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while Glycine N-methyltransferase, glycine-''N''-methyl transferase generates sarcosine from glycine. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adolph Strecker
Adolph Strecker (21 October 1822 – 7 November 1871) was a German chemist who is known primarily for his work with amino acids. Life and work Strecker was born in Darmstadt, the son of Friedrich Ludwig Strecker, an archivist working for the hessian Grand Duke, and Henriette Amalie Johannette Koch. Adolph Strecker attended school in Darmstadt until 1838 when he changed to the higher Gewerbeschule. After receiving his abitur in 1840, Strecker began studying science at the University of Giessen, where Justus Liebig was a professor. In August 1842, Strecker received his PhD and began teaching at a realschule in Darmstadt. He refused one offer to work for Liebig, but in 1846 he accepted another and became Liebig's private assistant at the University of Giessen. Strecker finished his habilitation in 1848 and became a lecturer at the university. Strecker investigated a wide variety of problems in both organic and inorganic chemistry during his time at Giessen. Examples include the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bloodstream
In vertebrates, the circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the body. It includes the cardiovascular system, or vascular system, that consists of the heart and blood vessels (from Greek meaning ''heart'', and Latin meaning ''vessels''). The circulatory system has two divisions, a systemic circulation or circuit, and a pulmonary circulation or circuit. Some sources use the terms ''cardiovascular system'' and ''vascular system'' interchangeably with ''circulatory system''. The network of blood vessels are the great vessels of the heart including large elastic arteries, and large veins; other arteries, smaller arterioles, capillaries that join with venules (small veins), and other veins. The circulatory system is closed in vertebrates, which means that the blood never leaves the network of blood vessels. Many invertebrates such as arthropods have an open circulatory system with a heart that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanidinoacetate N-methyltransferase
Guanidinoacetate N-methyltransferase () is an enzyme that catalyzes the chemical reaction and is encoded by gene ''GAMT'' located on chromosome 19p13.3. :S-adenosyl-L-methionine + guanidinoacetate \rightleftharpoons S-adenosyl-L-homocysteine + creatine Thus, the two substrates of this enzyme are S-adenosyl methionine ''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur thro ... and guanidinoacetate, whereas its two product (chemistry), products are S-adenosylhomocysteine and creatine. This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The List of enzymes, systematic name of this enzyme class is S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase. Other names in common use include GA methylpherase, guanidinoacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
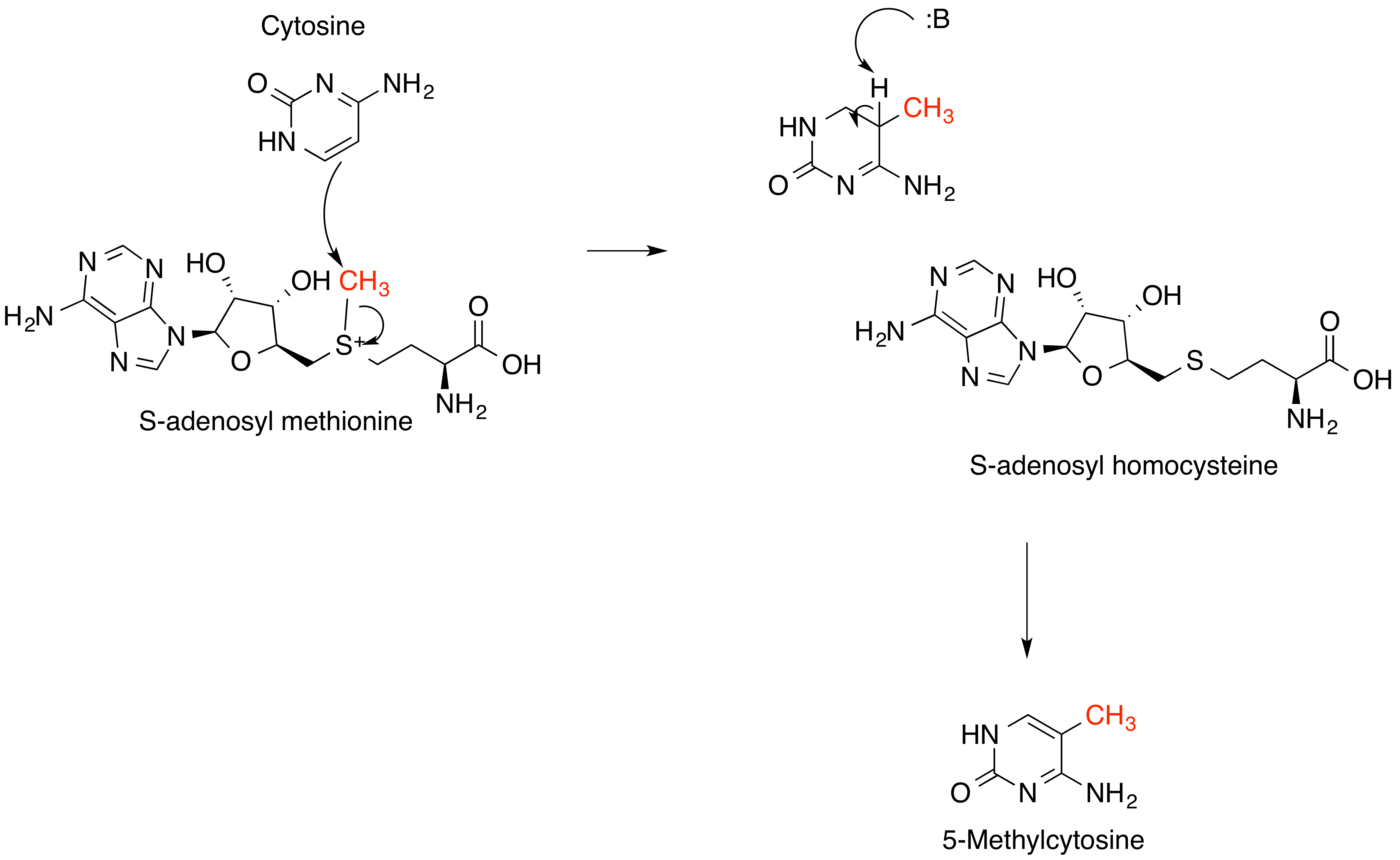
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GAA Biosynthesis Pathway
Gaa may refer to: * Gaa language, a language of Nigeria * gaa, the ISO 639 code for the Ga language of Ghana GAA may stand for: Compounds * Glacial (water-free), acetic acid * Acid alpha-glucosidase, also known as glucosidase, alpha; acid, an enzyme * a codon for glutamic acid Government * GADA 601, Argentine Army unit ''Grupo de Artillería Antiaérea 601'' (Anti-Aircraft Artillery Group 601) * General Allotment Act, US law passed in 1887 regarding Indian land * Group Areas Acts, South African apartheid laws * General Appropriation Act, the legislative act used in some countries for a national or state budget Organisations * Gaelic Athletic Association, governing body of Gaelic games such as hurling and Gaelic football * Gay Activists Alliance, New York City gay rights organisation 1969–81 * Gemmological Association of Australia, an educational organisation based in Sydney * Global Accounting Alliance, an accounting organisation * Global Aquaculture Alliance, an internatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrulline
The organic compound citrulline is an α-amino acid. Its name is derived from '' citrullus'', the Latin word for watermelon. Although named and described by gastroenterologists since the late 19th century, it was first isolated from watermelon in 1914 by Japanese researchers Yatarō Koga (古賀彌太郎) and Ryō Ōtake (大嶽了) and further codified by Mitsunori Wada of Tokyo Imperial University in 1930. It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase. Biosynthesis Citrulline can be derived from: * from arginine via nitric oxide synthase, as a byproduct of the production of nitric oxide for signaling purposes * from ornithine through the breakdown of proline or glutamine/glutamate * from asymmetric dimet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic. The urea cycle converts highly toxic ammonia to urea for excretion. This cycle was the first metabolic cycle to be discovered by Hans Krebs and Kurt Henseleit in 1932, five years before the discovery of the TCA cycle. The urea cycle was described in more detail later on by Ratner and Cohen. The urea cycle takes place primarily in the liver and, to a lesser extent, in the kidneys. Function Amino acid catabolism results in waste ammonia. All animals need a way to excrete this product. Most aquatic organisms, or ammonotelic organisms, excrete ammonia without converting it. Organisms that cannot easily and safely remove nitrogen as ammonia convert it to a less toxic substance, such as urea, via the urea cycle, which occurs mainly in the liver. Urea produced by the li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine
Ornithine is a non-proteinogenic α-amino acid that plays a role in the urea cycle. It is not incorporated into proteins during translation. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency, a disorder of the urea cycle. The Moiety (chemistry), moiety derived from ornithine is called ornithyl. Role in urea cycle L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central component of the urea cycle, which enables the disposal of excess nitrogen. Ornithine itself is recycled and, in a sense, acts as a catalyst. First, ammonia is converted into carbamoyl phosphate () by carbamoyl phosphate synthetase. Ornithine transcarbamylase then catalyzes the reaction between carbamoyl phosphate and ornithine to form citrulline and phosphate (Pi). Another amino group is contributed by aspartate, leading to the formation of arginine and the byproduct fumarate. The resulting arginine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine Amidinotransferase
L-Arginine:glycine amidinotransferase (AGAT; ) is the enzyme that catalyses the transfer of an amidino group from L-arginine to glycine. The products are L-ornithine and glycocyamine, also known as guanidinoacetate, the immediate precursor of creatine. Creatine and its phosphorylated form play a central role in the energy metabolism of muscle and nerve tissues. Creatine is in highest concentrations in the skeletal muscle, heart, spermatozoa and photoreceptor cells. Creatine helps buffer the rapid changes in ADP/ ATP ratio in muscle and nerve cells during active periods. Creatine is also synthesized in other tissues, such as pancreas, kidneys, and liver, where amidinotransferase is located in the cytoplasm, including the intermembrane space of the mitochondria, of the cells that make up those tissues. Function L-Arginine:glycine amidinotransferase catalyses the first, which is also the committed step in the formation of creatine. The second step of the process, producing the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experiencing renal failure. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine. Structure Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a group. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule. In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction. Production Guanidine can be obtained from natural sources, being first isolated in 1861 by Adolph Strecker via the oxidative degradation of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |