|
Glycocyamine
Glycocyamine (or guanidinoacetate) is a metabolite of glycine in which the amino group has been converted into a guanidine by guanylation (transfer of a guanidine group from arginine). In vertebrate organism it is then transformed into creatine by methylation. Glycocyamine is used as a supplement and as a feed additive in poultry farming. However, the metabolism of creatine from glycocyamine in the liver causes a depletion of methyl groups. This causes homocysteine levels to rise, which has been shown to produce cardiovascular and skeletal problems. Glycocyamine plays a role in the metabolism of the amino acids serine, threonine, and proline. Production Biochemical synthesis Glycocyamine is formed in the mammalian organism primarily in the Kidney, kidneys by transferring the Guanidine, guanidine group of L-arginine by the enzyme Arginine:glycine amidinotransferase, L-Arg:Gly-amidinotransferase (AGAT) to the amino acid glycine. From L-arginine, ornithine is thus produced, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcosine
Sarcosine, also known as ''N''-methylglycine, or monomethylglycine, is a amino acid with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine. Sarcosine is ubiquitous in biological materials. It is used in manufacturing biodegradable surfactants and toothpastes as well as in other applications. It is also a reagent in organic synthesis. Sarcosine is sweet to the taste. Biochemistry Sarcosine is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while Glycine N-methyltransferase, glycine-''N''-methyl transferase generates sarcosine from glycine. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanidinopropionic Acid
β-Guanidinopropionic acid, also referred to as guanidinopropionic acid, beta-guanidinopropionic acid or β-GPA, is a dietary supplement A dietary supplement is a manufactured product intended to supplement a person's diet by taking a pill (pharmacy), pill, capsule (pharmacy), capsule, tablet (pharmacy), tablet, powder, or liquid. A supplement can provide nutrients eithe .... β-Guanidinopropionic acid, also known as Ompenaclid (RGX-202), is being investigated in colorectal cancer by Inspirna and Merck β-Guanidinopropionic acid is a white crystalline powder soluble in water (50 mg/ml-clear, colorless solution). Studies on animals (rats, monkeys, hamsters) show that acidic guanidine derivatives such as β-GPA can ameliorate hyperglycemia in animal models of noninsulin-dependent diabetes. Though the oral availability of β-GPA is well established, the basic uptake mechanism has not been studied yet. References {{reflist Guanidines Carboxylic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creatine
Creatine ( or ) is an organic compound with the nominal formula . It exists in various tautomers in solutions (among which are neutral form and various zwitterionic forms). Creatine is found in vertebrates, where it facilitates recycling of adenosine triphosphate (ATP), primarily in muscle and brain tissue. Recycling is achieved by converting adenosine diphosphate (ADP) back to ATP via donation of phosphate groups. Creatine also acts as a Buffer solution, buffer. History Creatine was first identified in 1832 when Michel Eugène Chevreul isolated it from the basified water-extract of skeletal muscle. He later named the crystallized precipitate after the Ancient Greek, Greek word for meat, ('). In 1928, creatine was shown to exist in Tautomer, equilibrium with creatinine. Studies in the 1920s showed that consumption of large amounts of creatine did not result in its excretion. This result pointed to the ability of the body to store creatine, which in turn suggested its use as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylglycine
Dimethylglycine (DMG) is a derivative of the amino acid glycine with the structural formula (CH3)2NCH2COOH. It can be found in beans and liver, and has a sweet taste. It can be formed from trimethylglycine upon the loss of one of its methyl groups. It is also a byproduct of the metabolism of choline. When DMG was first discovered, it was referred to as Vitamin B16, but, unlike true B vitamins, deficiency of DMG in the diet does not lead to any ill-effects and it is synthesized by the human body in the citric acid cycle meaning it does not meet the definition of a vitamin. Uses Dimethylglycine has been suggested for use as an athletic performance enhancer, immunostimulant, and a treatment for autism, epilepsy, or mitochondrial disease. There is no evidence that dimethylglycine is effective for treating mitochondrial disease. Published studies on the subject have shown little to no difference between DMG treatment and placebo in autism spectrum disorders. Biological activity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creatine
Creatine ( or ) is an organic compound with the nominal formula . It exists in various tautomers in solutions (among which are neutral form and various zwitterionic forms). Creatine is found in vertebrates, where it facilitates recycling of adenosine triphosphate (ATP), primarily in muscle and brain tissue. Recycling is achieved by converting adenosine diphosphate (ADP) back to ATP via donation of phosphate groups. Creatine also acts as a Buffer solution, buffer. History Creatine was first identified in 1832 when Michel Eugène Chevreul isolated it from the basified water-extract of skeletal muscle. He later named the crystallized precipitate after the Ancient Greek, Greek word for meat, ('). In 1928, creatine was shown to exist in Tautomer, equilibrium with creatinine. Studies in the 1920s showed that consumption of large amounts of creatine did not result in its excretion. This result pointed to the ability of the body to store creatine, which in turn suggested its use as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bloodstream
In vertebrates, the circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the body. It includes the cardiovascular system, or vascular system, that consists of the heart and blood vessels (from Greek meaning ''heart'', and Latin meaning ''vessels''). The circulatory system has two divisions, a systemic circulation or circuit, and a pulmonary circulation or circuit. Some sources use the terms ''cardiovascular system'' and ''vascular system'' interchangeably with ''circulatory system''. The network of blood vessels are the great vessels of the heart including large elastic arteries, and large veins; other arteries, smaller arterioles, capillaries that join with venules (small veins), and other veins. The circulatory system is closed in vertebrates, which means that the blood never leaves the network of blood vessels. Many invertebrates such as arthropods have an open circulatory system with a heart that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adolph Strecker
Adolph Strecker (21 October 1822 – 7 November 1871) was a German chemist who is known primarily for his work with amino acids. Life and work Strecker was born in Darmstadt, the son of Friedrich Ludwig Strecker, an archivist working for the hessian Grand Duke, and Henriette Amalie Johannette Koch. Adolph Strecker attended school in Darmstadt until 1838 when he changed to the higher Gewerbeschule. After receiving his abitur in 1840, Strecker began studying science at the University of Giessen, where Justus Liebig was a professor. In August 1842, Strecker received his PhD and began teaching at a realschule in Darmstadt. He refused one offer to work for Liebig, but in 1846 he accepted another and became Liebig's private assistant at the University of Giessen. Strecker finished his habilitation in 1848 and became a lecturer at the university. Strecker investigated a wide variety of problems in both organic and inorganic chemistry during his time at Giessen. Examples include the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanidinoacetate N-methyltransferase
Guanidinoacetate N-methyltransferase () is an enzyme that catalyzes the chemical reaction and is encoded by gene ''GAMT'' located on chromosome 19p13.3. :S-adenosyl-L-methionine + guanidinoacetate \rightleftharpoons S-adenosyl-L-homocysteine + creatine Thus, the two substrates of this enzyme are S-adenosyl methionine ''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur thro ... and guanidinoacetate, whereas its two product (chemistry), products are S-adenosylhomocysteine and creatine. This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The List of enzymes, systematic name of this enzyme class is S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase. Other names in common use include GA methylpherase, guanidinoacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
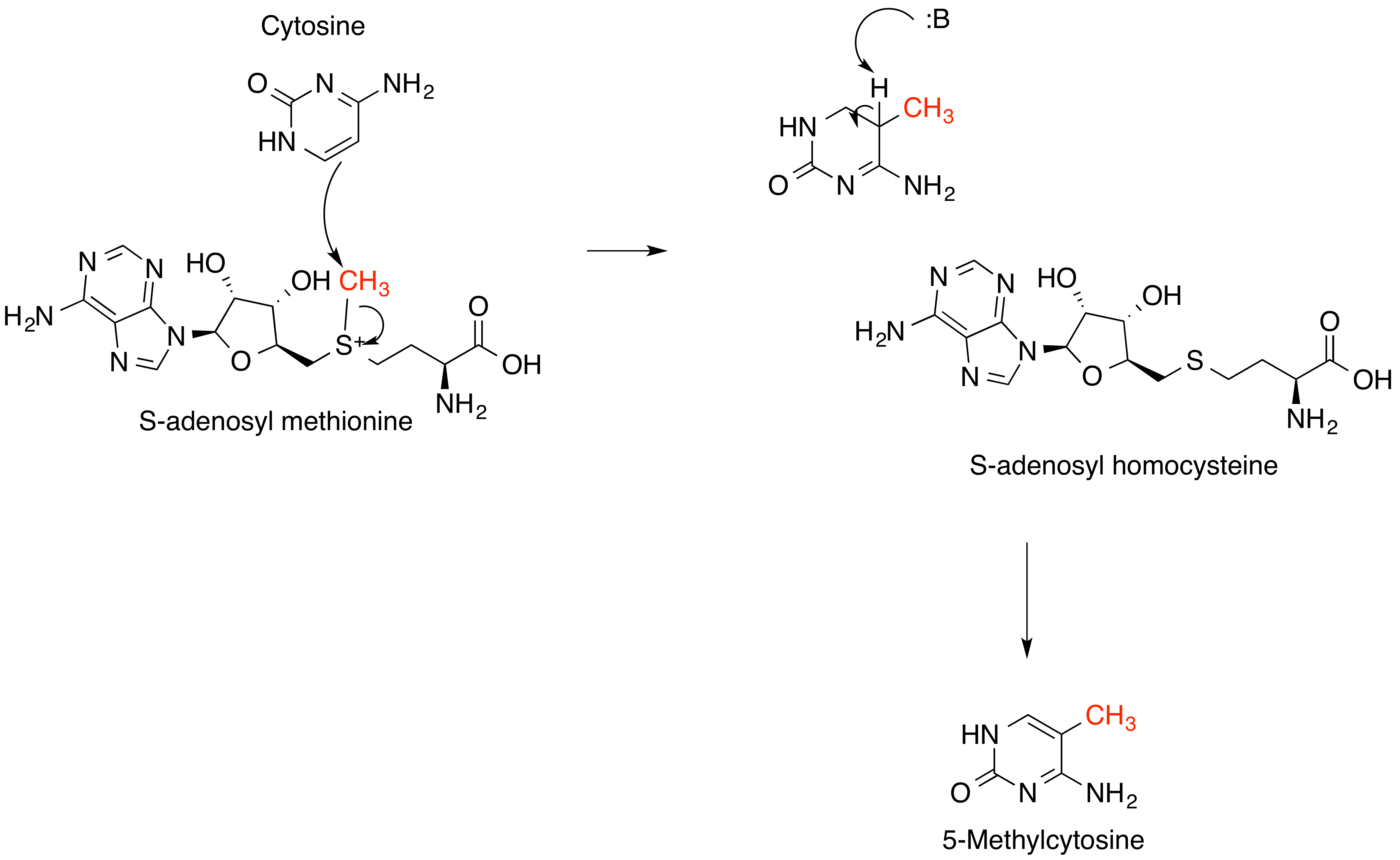
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrulline
The organic compound citrulline is an α-amino acid. Its name is derived from '' citrullus'', the Latin word for watermelon. Although named and described by gastroenterologists since the late 19th century, it was first isolated from watermelon in 1914 by Japanese researchers Yatarō Koga (古賀彌太郎) and Ryō Ōtake (大嶽了) and further codified by Mitsunori Wada of Tokyo Imperial University in 1930. It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia by converting it into urea. Citrulline is also produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase. Biosynthesis Citrulline can be derived from: * from arginine via nitric oxide synthase, as a byproduct of the production of nitric oxide for signaling purposes * from ornithine through the breakdown of proline or glutamine/glutamate * from asymmetric dimet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic. The urea cycle converts highly toxic ammonia to urea for excretion. This cycle was the first metabolic cycle to be discovered by Hans Krebs and Kurt Henseleit in 1932, five years before the discovery of the TCA cycle. The urea cycle was described in more detail later on by Ratner and Cohen. The urea cycle takes place primarily in the liver and, to a lesser extent, in the kidneys. Function Amino acid catabolism results in waste ammonia. All animals need a way to excrete this product. Most aquatic organisms, or ammonotelic organisms, excrete ammonia without converting it. Organisms that cannot easily and safely remove nitrogen as ammonia convert it to a less toxic substance, such as urea, via the urea cycle, which occurs mainly in the liver. Urea produced by the li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |