|
Fluvastatin
Fluvastatin is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular disease. It was patented in 1982 and approved for medical use in 1994. It is on the World Health Organization's List of Essential Medicines. Adverse effects Adverse effects are comparable to other statins. Common are nausea, indigestion, insomnia and headache. Myalgia (muscle pain), and rarely rhabdomyolysis, characteristic side effects for statins, can also occur. Interactions Contrary to lovastatin, simvastatin and atorvastatin, fluvastatin has no relevant interactions with drugs that inhibit the liver enzyme CYP3A4, and a generally lower potential for interactions than most other statins. Fluconazole, a potent inhibitor of CYP2C9, does increase fluvastatin levels. Pharmacology Mechanism of action Fluvastatin works by blocking the liver enzyme HMG-CoA reductase, which facilitates an important step in cholesterol synthesis. Pharmacodynamics In a Coc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statin
Statins (or HMG-CoA reductase inhibitors) are a class of medications that lower cholesterol. They are prescribed typically to people who are at high risk of cardiovascular disease. Low-density lipoprotein (LDL) carriers of cholesterol play a key role in the development of atherosclerosis and coronary heart disease via the mechanisms described by the lipid hypothesis. As lipid-lowering medications, statins are effective in lowering LDL cholesterol; they are widely used for primary prevention in people at high risk of cardiovascular disease, as well as in secondary prevention for those who have developed cardiovascular disease. Side effects of statins include muscle pain, increased risk of diabetes, and abnormal blood levels of certain liver enzymes. Additionally, they have rare but severe adverse effects, particularly muscle damage, and very rarely rhabdomyolysis. They act by inhibiting the enzyme HMG-CoA reductase, which plays a central role in the production of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
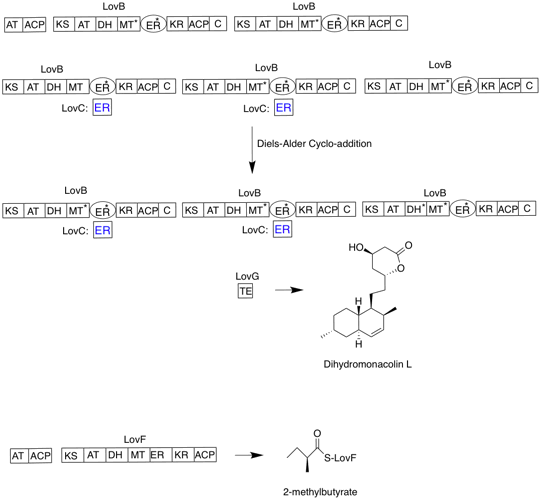
Lovastatin
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth. Common side effects include diarrhea, constipation, headache, muscles pains, rash, and trouble sleeping. Serious side effects may include liver problems, muscle breakdown, and kidney failure. Use during pregnancy may harm the baby and use during breastfeeding is not recommended. It works by decreasing the liver's ability to produce cholesterol by blocking the enzyme HMG-CoA reductase. Lovastatin was patented in 1979 and approved for medical use in 1987. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. In 2022, it was the 111th most commonly prescribed medication in the United States, with more than 5million prescriptions. Medical uses The primary uses of lovastatin is f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diols
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Classification Pharmaceutical drugs are often classified into drug classes—groups of related drugs that hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Protein Binding
Plasma protein binding refers to the degree to which medications attach to blood proteins within the blood plasma. A drug's efficacy may be affected by the degree to which it binds. The less bound a drug is, the more efficiently it can traverse or diffuse through cell membranes. Common blood proteins that drugs bind to are human serum albumin, lipoprotein, glycoprotein, and α, β‚ and γ globulins. Binding (drug distribution) A drug in blood exists in two forms: bound and unbound. Depending on a specific drug's affinity for plasma proteins, a proportion of the drug may become bound to the proteins, with the remainder being unbound. If the protein binding is reversible, then a chemical equilibrium will exist between the bound and unbound states, such that: :Protein + drug ⇌ Protein-drug complex Notably, it is the unbound fraction which exhibits pharmacologic effects. It is also the fraction that may be metabolized and/or excreted. For example, the "fraction bound" of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Pass Effect
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism at a specific location in the body which leads to a reduction in the concentration of the active drug before it reaches the site of action or systemic circulation. The effect is most associated with orally administered medications, but some drugs still undergo first-pass metabolism even when delivered via an alternate route (e.g., IV, IM, etc.). During this metabolism, drug is lost during the process of absorption which is generally related to the liver and gut wall. The liver is the major site of first pass effect; however, it can also occur in the lungs, vasculature or other metabolically active tissues in the body. Notable drugs that experience a significant first pass effect are buprenorphine, chlorpromazine, cimetidine, diazepam, ethanol (drinking alcohol), imipramine, insulin, lidocaine, midazolam, morphine, pethidine, propranolol, and tetrahydroca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |