|
Cyclopentanone
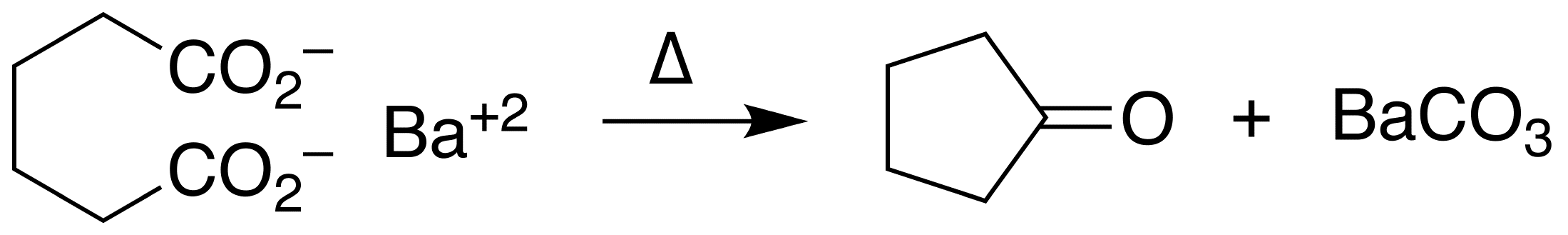
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid. Preparation Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopentanone: :(CH2)4(CO2H)2 → (CH2)4CO + H2O + CO2 Uses Cyclopentanone is common precursor to fragrances, especially those related to jasmine and jasmone Jasmone is an organic compound, which is a volatile portion of the oil from jasmine flowers. It is a colorless to pale yellow liquid. Jasmone can exist in two isomeric forms with differing geometry around the pentenyl double bond, ''cis''-jasmon .... Examples include 2-pentyl- and 2-heptylcyclopentanone.Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim. It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital. Cyclopentanone is also used to mak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentenone
2-Cyclopentenone is a ketone with chemical formula and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid. The term cyclopentenone may also refer to a structural motif wherein the cyclopentenone moiety is a subunit of a larger molecule. Cyclopentenones are found in a large number of natural products, including jasmone, the aflatoxins, and several prostaglandins. Synthesis 2-Cyclopentenones can be synthesized in a number of ways. One of the routes involves elimination of α- bromo- cyclopentanone using lithium carbonate and Claisen condensation-decarboxylation- isomerization cascades of unsaturated diesters as shown below. The acid-catalyzed dehydration of cyclopentanediols affords cyclopentenone. As a functional group, the synthesis of 2-cyclopentenones is accomplished in a variety of ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketonization
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat with expulsion of one equivalent of water () and one equivalent of carbon dioxide (): :\ce\mathbf + \ce\mathbf \longrightarrow \ce\mathbf + \ce Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl (), possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported. This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is charact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentethylcyclanone
Pentethylcyclanone is an antitussive medication A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field an ... having molecular formula C16H25NO2 . Synthesis Pentethylcyclanone can be prepared by alkylation of the anion of the self-condensation product of cyclopentanone with ''N''-(2-chloroethyl)-morpholine. References {{Cough and cold preparations Expectorants 4-Morpholinyl compunds Cyclopentanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Hydroxide
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (''x'' = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form. Preparation and structure Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water: :BaO + H2O → Ba(OH)2 It crystallises as the octahydrate, which converts to the monohydrate upon heating in air. At 100 °C in a vacuum, the monohydrate will yield BaO and water. The monohydrate adopts a layered structure (see picture above). The Ba2+ centers adopt a square anti-prismatic geometry. Each Ba2+ center is bound by two water ligands and six hydroxide ligands, which are respectively doubly and triply bridging to neighboring Ba2+ centre sites. In the octahydrate, the individual Ba2+ centers are again eight coordinate but do not share ligands. Uses Industrially, barium hydroxide is used as the precursor to other bari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adipic Acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Preparation and reactivity Adipic acid is produced from a mixture of cyclohexanone and cyclohexanol called KA oil, the abbreviation of ketone-alcohol oil. The KA oil is oxidized with nitric acid to give adipic acid, via a multistep pathway. Early in the reaction, the cyclohexanol is converted to the ketone, releasing nitrous acid: :HOC6H11 + HNO3 → OC(CH2)5 + HNO2 + H2O Among its many reactions, the cyclohexanone is nitrosated, setting the stage for the scission of the C-C bond: :HNO2 + HNO3 → NO+NO3− + H2O :OC6H10 + NO+ → OC6H9-2-NO + H+ Side produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentobarbital
Cyclopentobarbital sodium (Cyclopal, Dormisan) is a barbiturate derivative invented in the 1940s. It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine. Cyclopal is considered similar in effects to phenobarbital but lasts almost three times as long, and is considered a long-acting barbiturate with a fairly slow onset of action. See also * Barbiturate Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as ... References Allyl compounds General anesthetics Barbiturates GABAA receptor positive allosteric modulators Cyclopentenes {{sedative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone Solvents
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars ( ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pencycuron
Pencycuron is a phenylurea fungicide developed by Bayer Crop Science and marketed under the brand name Monceren. It has specific activity against the plant pathogen ''Rhizoctonia solani ''Rhizoctonia solani'' is a species of fungus in the order Cantharellales. Basidiocarps (fruit bodies) are thin, effused, and web-like, but the fungus is more typically encountered in its anamorphic state, as hyphae and sclerotia. The name ''Rhi ...'' for which it was developed. References External links * Fungicides Ureas Cyclopentyl compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentamine
Cyclopentamine (trade names Clopane, Cyclonarol, Cyclosal, Cyklosan, Nazett, Sinos, among others) is a sympathomimetic alkylamine, classified as a vasoconstrictor. Cyclopentamine was indicated in the past as an over-the-counter (OTC) medication for use as a nasal decongestant, notably in Europe and Australia, but has now been largely discontinued. Pharmacology Cyclopentamine acts as a releasing agent of the catecholamine neurotransmitters norepinephrine (noradrenaline), epinephrine (adrenaline), and dopamine. Its effects on norepinephrine and epinephrine mediate its decongestant effects, while its effects on all three neurotransmitters are responsible for its stimulant properties. When ingested orally in sufficient quantities, cyclopentamine produces similar effects to amphetamine, methamphetamine, and propylhexedrine. Chemistry Cyclopentamine is the cyclopentane homolog of propylhexedrine, differing only in terms of the contracted ring size of a cyclopentane, co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopal
Cyclopentobarbital sodium (Cyclopal, Dormisan) is a barbiturate derivative invented in the 1940s. It has sedative and anticonvulsant properties, and was used primarily as an anaesthetic in veterinary medicine. Cyclopal is considered similar in effects to phenobarbital but lasts almost three times as long, and is considered a long-acting barbiturate with a fairly slow onset of action. See also * Barbiturate Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as we ... References Allyl compounds General anesthetics Barbiturates GABAA receptor positive allosteric modulators Cyclopentenes {{sedative-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monograph on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an appendix with monographs on organic named reactions. The 15th edition was published in A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |