|
Consalazinic Acid
Consalazinic acid is a chemical compound with the molecular formula . It is classified as a depsidone and is a secondary metabolite produced by a variety of lichen A lichen ( , ) is a hybrid colony (biology), colony of algae or cyanobacteria living symbiotically among hypha, filaments of multiple fungus species, along with yeasts and bacteria embedded in the cortex or "skin", in a mutualism (biology), m ...s. Consalazinic acid was first isolated from '' Parmotrema subisidiosum'' and described in 1980. It has since been identified in many other lichens. References Lactones Lichen products Polyphenols Heterocyclic compounds with 4 rings Benzodioxepines Benzofurans Hydroxymethyl compounds Oxygen heterocycles {{Ether-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Depsidone
Depsidones (+ " depside" + "one") are chemical compounds that are sometimes found as secondary metabolites in lichens. They are esters that are both depsides and cyclic ethers. An example is norstictic acid Norstictic acid is a depsidone produced as a secondary metabolites in lichens. The compound contains both an aldehyde carbonyl group and an adjacent hydroxyl In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical fo .... References {{reflist Biochemistry Carboxylate esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Secondary Metabolite
Secondary metabolites, also called ''specialised metabolites'', ''secondary products'', or ''natural products'', are organic compounds produced by any lifeform, e.g. bacteria, archaea, fungi, animals, or plants, which are not directly involved in the normal cell growth, growth, Biological development, development, or reproduction of the organism. Instead, they generally mediate ecological biological interaction, interactions, which may produce a Natural selection, selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs. The term secondary metabolite was first coined by Albrecht Kossel, the 1910 Nobel Prize laureate for medicine and physio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lichen
A lichen ( , ) is a hybrid colony (biology), colony of algae or cyanobacteria living symbiotically among hypha, filaments of multiple fungus species, along with yeasts and bacteria embedded in the cortex or "skin", in a mutualism (biology), mutualistic relationship.Introduction to Lichens – An Alliance between Kingdoms . University of California Museum of Paleontology. . Lichens are the lifeform that first brought the term symbiosis (as ''Symbiotismus'') into biological context. Lichens have since been recognized as important actors in nutrient cycling and producers which many higher trophic feeders feed on, such as reindeer, gastropods, nematodes, mites, and springtails. Lichens have properties different from those of their component organisms. They come in man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Parmotrema Subisidiosum
''Parmotrema'' is a genus of lichen belonging to the family Parmeliaceae. It is a large genus, containing an estimated 300 species, with a centre of diversity in subtropical regions of South America and the Pacific Islands. Members of the genus are commonly called ruffle lichens or scatter-rag lichens.Field Guide to California Lichens, Stephen Sharnoff, Yale University Press, 2014, Description ''Parmotrema'' is characterized by its typically large, moderately to loosely-attached foliose thallus with broad lobes that are usually more than 5 mm wide. There is a broad, naked zone around the margin of the lower surface, an epicortex with pores and an upper cortex with a palisade-plectenchymatous arrangement of hyphae. Ascospores are thick-walled and ellipsoid. Taxonomy ''Parmotrema'' was proposed as a genus by Italian lichenologist Abramo Bartolommeo Massalongo in 1860, with ''Parmotrema perforatum'' as the type species. The genus name, composed of the Greek ''parmos'' (cup) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lactones
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lactone = 4-membered, γ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lichen Products
Lichen products, also known as lichen substances, are organic compounds produced by a lichen. Specifically, they are secondary metabolites. Lichen products are represented in several different chemical classes, including terpenoids, orcinol derivatives, chromones, xanthones, depsides, and depsidones. Over 800 lichen products of known chemical structure have been reported in the scientific literature, and most of these compounds are exclusively found in lichens. Examples of lichen products include usnic acid (a dibenzofuran), atranorin (a depside), lichexanthone (a xanthone), salazinic acid (a depsidone), and isolichenan, an α-glucan. Many lichen products have biological activity, and research into these effects is ongoing. Biosynthesis Most lichen products are biochemically synthesized via the acetyl-polymalonyl pathway (also known as polyketide pathway), while only a few originate from the mevalonate and shikimate biosynthetic pathways. Occurrence Lichen products accumulate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyphenols
Polyphenols () are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments. Etymology The name derives from the Ancient Greek word (, meaning "many, much") and the word ‘phenol’ which refers to a chemical structure formed by attachment of an aromatic benzenoid (phenyl) ring to a hydroxyl (-OH) group (hence the ''-ol'' suffix). The term "polyphenol" has been in use at least since 1894. Definition Polyphenols are natural products with "one or several hydroxyl groups on aromatic rings", including four principal classes: phenolic acids, flavonoids, stilbenes, and lignans. Flavonoids can be grouped as flavones, flavonols, flavanols, flavanones, isoflavones, proanthocyanidins, and anthocyanins. Particularly abundant flavanoids in foods are catechin (tea, fruits), hespereti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heterocyclic Compounds With 4 Rings
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Benzofurans
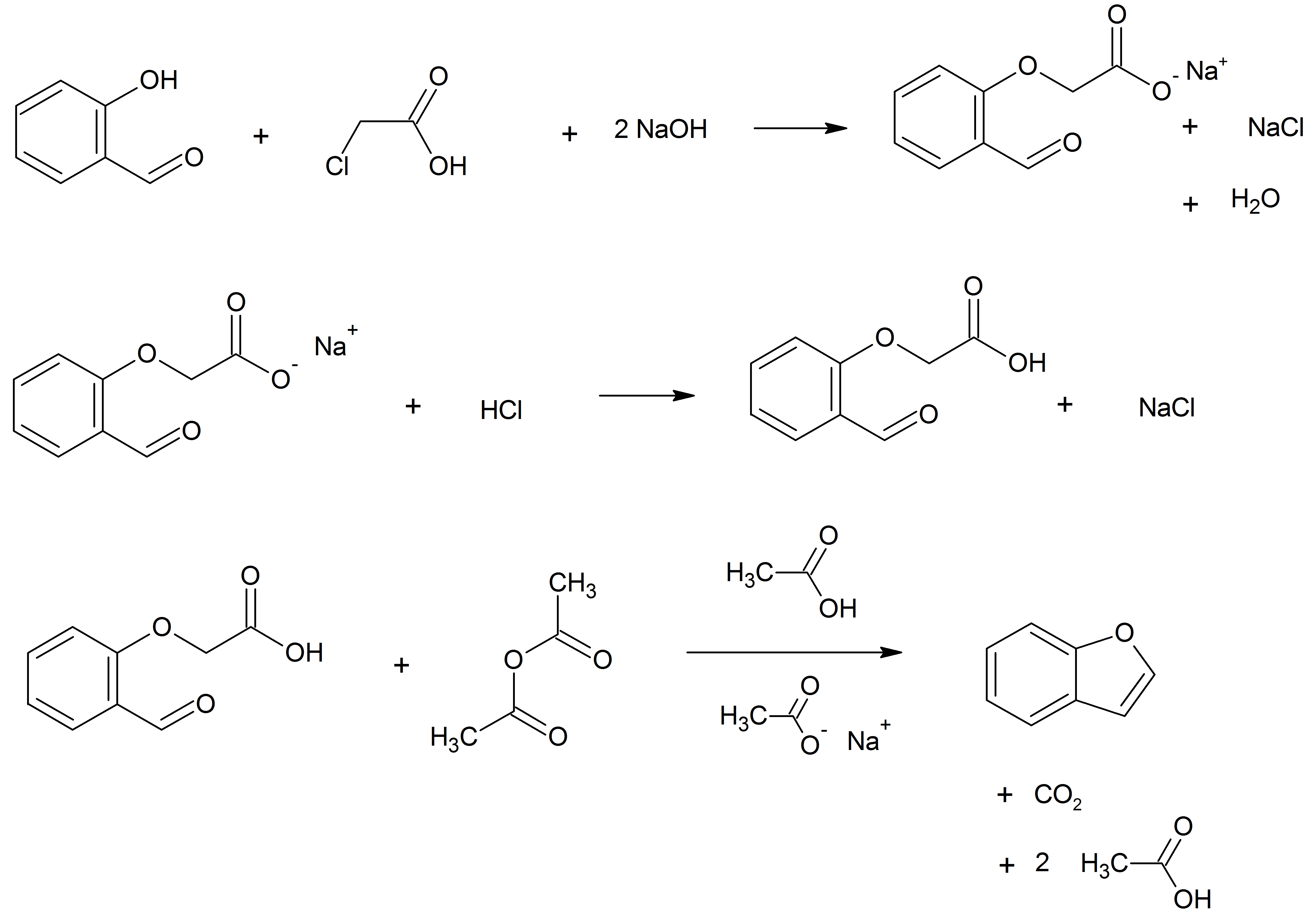
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration reaction, dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of Nitroalkene, nitro vinyl furans with various dienophiles: : *Isomerization, Cycloisomerization of alkyne Arene substitution pattern, ortho-substituted phenols: : Related compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydroxymethyl Compounds
The hydroxymethyl group is a substituent with the structural formula . It consists of a methylene bridge ( unit) bonded to a hydroxyl group (). This makes the hydroxymethyl group an alcohol. It has the identical chemical formula with the methoxy group () that differs only in the attachment site and orientation to the rest of the molecule. However, their chemical properties are different. Hydroxymethyl is the side chain of encoded amino acid serine Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − .... References External links {{Organic-compound-stub Functional groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |