 |
Bulk Modulus
The bulk modulus (K or B or k) of a substance is a measure of the resistance of a substance to bulk compression. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume. Other moduli describe the material's response ( strain) to other kinds of stress: the shear modulus describes the response to shear stress, and Young's modulus describes the response to normal (lengthwise stretching) stress. For a fluid, only the bulk modulus is meaningful. For a complex anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behaviour, and one must use the full generalized Hooke's law. The reciprocal of the bulk modulus at fixed temperature is called the isothermal compressibility. Definition The bulk modulus K (which is usually positive) can be formally defined by the equation :K=-V\frac , where P is pressure, V is the initial volume of the substance, and dP/dV deno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Isostatic Pressure Deformation structures in physics and engineering
{{disambig ...
The term isostatic may refer to: * Isostatic depression in geodynamics * Isostatic powder compaction in metallurgy and ceramic engineering * Isostatic press in manufacturing See also *Isostasy in geology: gravitational equilibrium between the Earth's lithosphere and asthenosphere *Statically determinate In statics and structural mechanics, a structure is statically indeterminate when the equilibrium equations force and moment equilibrium conditions are insufficient for determining the internal forces and reactions on that structure. Mathemat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Pounds Per Square Inch
The pound per square inch (abbreviation: psi) or, more accurately, pound-force per square inch (symbol: lbf/in2), is a unit of measurement of pressure or of stress based on avoirdupois units and used primarily in the United States. It is the pressure resulting from a force with magnitude of one pound-force applied to an area of one square inch. In SI units, 1 psi is approximately . The pound per square inch absolute (psia) is used to make it clear that the pressure is relative to a vacuum rather than the ambient atmospheric pressure. Since atmospheric pressure at sea level is around , this will be added to any pressure reading made in air at sea level. The converse is pound per square inch gauge (psig), indicating that the pressure is relative to atmospheric pressure. For example, a bicycle tire pumped up to 65 psig in a local atmospheric pressure at sea level (14.7 psi) will have a pressure of 79.7 psia (14.7 psi + 65 psi). When gauge pressure is referenced to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Powder Diffraction
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer. Powder diffraction stands in contrast to single crystal diffraction techniques, which work best with a single, well-ordered crystal. Explanation The most common type of powder diffraction is with X-rays, the focus of this article, although some aspects of neutron powder diffraction are mentioned. (Powder electron diffraction is more complex due to dynamical diffraction and is not discussed further herein.) Typical diffractometers use electromagnetic radiation (waves) with known wavelength and frequency, which is determined by their source. The source is often X-rays, and neutrons are also common sources, with their frequency determined by their de Broglie wavelength. When these waves reach the sample, the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Elastic Modulus
An elastic modulus (also known as modulus of elasticity (MOE)) is a quantity that describes an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. Definition The elastic modulus of an object is defined as the slope of its stress–strain curve in the elastic deformation region: A stiffer material will have a higher elastic modulus. An elastic modulus has the form: :\delta \ \stackrel\ \frac where stress is the force causing the deformation divided by the area to which the force is applied and strain is the ratio of the change in some parameter caused by the deformation to the original value of the parameter. Since strain is a dimensionless quantity, the units of \delta will be the same as the units of stress. Elastic constants and moduli Elastic constants are specific parameters that quantify the stiffness of a material in response to applied stresses and are fundamental in defining the elastic pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
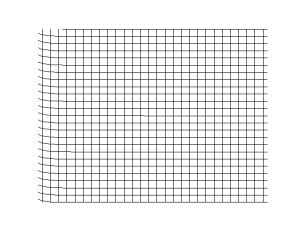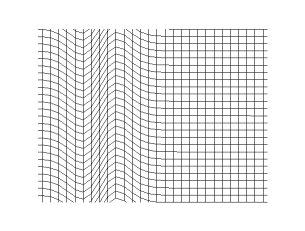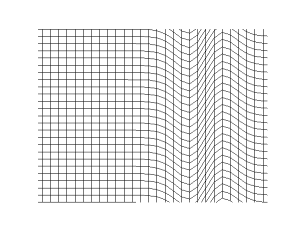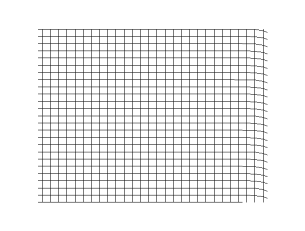 |
Transverse Waves
In physics, a transverse wave is a wave that oscillates perpendicularly to the direction of the wave's advance. In contrast, a longitudinal wave travels in the direction of its oscillations. All waves move energy from place to place without transporting the matter in the transmission medium if there is one. Electromagnetic waves are transverse without requiring a medium. The designation “transverse” indicates the direction of the wave is perpendicular to the displacement of the particles of the medium through which it passes, or in the case of EM waves, the oscillation is perpendicular to the direction of the wave. A simple example is given by the waves that can be created on a horizontal length of string by anchoring one end and moving the other end up and down. Another example is the waves that are created on the membrane of a drum. The waves propagate in directions that are parallel to the membrane plane, but each point in the membrane itself gets displaced up and down, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
P-wave
A P wave (primary wave or pressure wave) is one of the two main types of elastic body waves, called seismic waves in seismology. P waves travel faster than other seismic waves and hence are the first signal from an earthquake to arrive at any affected location or at a seismograph. P waves may be transmitted through gases, liquids, or solids. Nomenclature The name ''P wave'' can stand for either pressure wave (as it is formed from alternating compressions and rarefactions) or primary wave (as it has high velocity and is therefore the first wave to be recorded by a seismograph). The name '' S wave'' represents another seismic wave propagation mode, standing for secondary or shear wave, a usually more destructive wave than the primary wave. Seismic waves in the Earth Primary and secondary waves are body waves that travel within the Earth. The motion and behavior of both P and S waves in the Earth are monitored to probe the interior structure of the Earth. Discontinuities ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Speed Of Sound
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elasticity (solid mechanics), elastic medium. More simply, the speed of sound is how fast vibrations travel. At , the speed of sound in air is about , or in or one mile in . It depends strongly on temperature as well as the medium through which a sound wave is propagating. At , the speed of sound in dry air (sea level 14.7 psi) is about . The speed of sound in an ideal gas depends only on its temperature and composition. The speed has a weak dependence on frequency and pressure in dry air, deviating slightly from ideal behavior. In colloquial speech, ''speed of sound'' refers to the speed of sound waves in Earth's atmosphere, air. However, the speed of sound varies from substance to substance: typically, sound travels most slowly in gases, faster in liquids, and fastest in solids. For example, while sound travels at in air, it travels at in water (almost 4.3 times a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Heat Capacity Ratio
In thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or Laplace's coefficient, is the ratio of the heat capacity at constant pressure () to heat capacity at constant volume (). It is sometimes also known as the '' isentropic expansion factor'' and is denoted by (gamma) for an ideal gasγ first appeared in an article by the French mathematician, engineer, and physicist Siméon Denis Poisson: * On p. 332, Poisson defines γ merely as a small deviation from equilibrium which causes small variations of the equilibrium value of the density ρ. In Poisson's article of 1823 – * γ was expressed as a function of density D (p. 8) or of pressure P (p. 9). Meanwhile, in 1816 the French mathematician and physicist Pierre-Simon Laplace had found that the speed of sound depends on the ratio of the specific heats. * However, he didn't denote the ratio as γ. In 1825, Laplace stated that the speed of sound i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Ideal Gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions. Under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules (or atoms for monatomic gas) play the role of the ideal particles. Many gases such as nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide and mixtures such as air, can be treated as ideal gases within reasonable tolerances over a considerable parameter range around standard temperature and pressure. Generally, a gas behaves more like an ideal gas at higher temperature and lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Isentropic Process
An isentropic process is an idealized thermodynamic process that is both Adiabatic process, adiabatic and Reversible process (thermodynamics), reversible. The work (physics), work transfers of the system are friction, frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamics, Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such. The word "isentropic" derives from the proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a concise d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |