|
Volume Fraction
In chemistry and fluid mechanics, the volume fraction φ''i'' is defined as the volume of a constituent ''V''''i'' divided by the volume of all constituents of the mixture ''V'' prior to mixing: :\phi_i = \frac Being dimensionless, its unit is 1; it is expressed as a number, e.g., 0.18. It is the same concept as volume percent (vol%) except that the latter is expressed with a denominator of 100, e.g., 18%. The volume fraction coincides with the volume concentration in ideal solutions where the volumes of the constituents are additive (the volume of the solution is equal to the sum of the volumes of its ingredients). The sum of all volume fractions of a mixture is equal to 1: :\sum_^ V_i = V ; \qquad \sum_^ \phi_i = 1 The volume fraction (percentage by volume, vol%) is one way of expressing the composition of a mixture with a dimensionless quantity; mass fraction (percentage by weight, wt%) and mole fraction (percentage by moles, mol%) are others. Volume concentration and vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions. Under various conditions of temperature and pressure, many real gases behave qualitatively like an ideal gas where the gas molecules (or atoms for monatomic gas) play the role of the ideal particles. Many gases such as nitrogen, oxygen, hydrogen, noble gases, some heavier gases like carbon dioxide and mixtures such as air, can be treated as ideal gases within reasonable tolerances over a considerable parameter range around standard temperature and pressure. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to resolve include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimensionless Numbers Of Chemistry
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1), ISBN 978-92-822-2272-0. which is not explicitly shown. Dimensionless quantities are widely used in many fields, such as mathematics, physics, chemistry, engineering, and economics. Dimensionless quantities are distinct from quantities that have associated dimensions, such as time (measured in seconds). Dimensionless units are dimensionless values that serve as units of measurement for expressing other quantities, such as radians (rad) or steradians (sr) for plane angles and solid angles, respectively. For example, optical extent is defined as having units of metres multiplied by steradians. History Quantities having dimension one, ''dimensionless quantities'', regularly occur in sciences, and are formally treated within t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percentage
In mathematics, a percentage (from la, per centum, "by a hundred") is a number or ratio expressed as a fraction of 100. It is often denoted using the percent sign, "%", although the abbreviations "pct.", "pct" and sometimes "pc" are also used. A percentage is a dimensionless number (pure number); it has no unit of measurement. Examples For example, 45% (read as "forty-five per cent") is equal to the fraction , the ratio 45:55 (or 45:100 when comparing to the total rather than the other portion), or 0.45. Percentages are often used to express a proportionate part of a total. (Similarly, one can also express a number as a fraction of 1,000, using the term "per mille" or the symbol "".) Example 1 If 50% of the total number of students in the class are male, that means that 50 out of every 100 students are male. If there are 500 students, then 250 of them are male. Example 2 An increase of $0.15 on a price of $2.50 is an increase by a fraction of = 0.06. Expressed as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
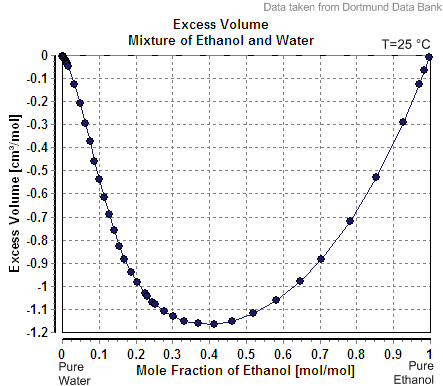
Excess Molar Quantity
In chemical thermodynamics, excess properties are properties of mixtures which quantify the non- ideal behavior of real mixtures. They are defined as the difference between the value of the property in a real mixture and the value that would exist in an ideal solution under the same conditions. The most frequently used excess properties are the excess volume, excess enthalpy, and excess chemical potential. The excess volume (), internal energy (), and enthalpy () are identical to the corresponding mixing properties; that is, :\begin V^E &= \Delta V_\text \\ H^E &= \Delta H_\text \\ U^E &= \Delta U_\text \end These relationships hold because the volume, internal energy, and enthalpy changes of mixing are zero for an ideal solution. Definition By definition, excess properties are related to those of the ideal solution by: :z^E = z - z^\text Here, the superscript IS denotes the value in the ideal solution, a superscript E denotes the excess molar property, and z denotes the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Molar Volume
In thermodynamics, a partial molar property is a quantity which describes the variation of an extensive property of a solution or mixture with changes in the molar composition of the mixture at constant temperature and pressure. It is the partial derivative of the extensive property with respect to the amount (number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property. Definition The partial molar volume is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is more to it than this: When one mole of water is added to a large volume of water at 25 °C, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol−1. However, addition of one mole of water to a large volume of pure ethanol results in an increase in volume of only 14 cm3. The reason that the increase is differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apparent Molar Property
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture. It shows the change in the corresponding solution property (for example, volume) per mole of that component added, when all of that component is added to the solution. It is described as ''apparent'' because it appears to represent the molar property of that component ''in solution'', provided that the properties of the other solution components are assumed to remain constant during the addition. However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state. For instance, the volume of a solution containing two components identified as solvent and solute is given by : V=V_0 + ^\phi_1 \ =\tilde_ n_ + ^\phi\tilde_1 n_1 \, where is the volume of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Proof
Alcohol proof (usually termed simply "proof" in relation to a beverage) is a measure of the content of ethanol (alcohol) in an alcoholic beverage. The term was originally used in England and was equal to about 1.8 times the percentage of alcohol by volume (ABV). The UK now uses ABV instead of proof. In the United States, alcohol proof is defined as twice the percentage of ABV. The definition of proof in terms of ABV varies from country to country. The measurement of alcohol content and the statement of content on bottles of alcoholic beverages is regulated by law in many countries. In 1972, Canada phased out the use of "proof"; in 1973, the European Union followed suit; and the UK, where the concept originated, started using ABV instead in 1980. The US code mandates the use of ABV, but permits proof to be used also. The degree symbol (°) is sometimes used to indicate alcohol proof. History The term ''proof'' dates back to 16th century England, when spirits were taxed at diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Meter
A breathalyzer or breathalyser (a portmanteau of ''breath'' and ''analyzer/analyser'') is a device for estimating blood alcohol content (BAC), or to detect viruses or diseases from a breath sample. The name is a genericized trademark of the Breathalyzer brand name of instruments developed by inventor Robert Frank Borkenstein in the 1950s. Origins A 1927 paper produced by Emil Bogen, who collected air in a football bladder and then tested this air for traces of alcohol, discovered that the alcohol content of 2 litres of expired air was a little greater than that of 1 cc of urine. However, research into the possibilities of using breath to test for alcohol in a person's body dates as far back as 1874, when Francis E. Anstie made the observation that small amounts of alcohol were excreted in breath. Also, in 1927 a Chicago chemist, William Duncan McNally, invented a breathalyzer in which the breath moving through chemicals in water would change color. One use for h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol By Volume
Alcohol by volume (abbreviated as ABV, abv, or alc/vol) is a standard measure of how much alcohol ( ethanol) is contained in a given volume of an alcoholic beverage (expressed as a volume percent). It is defined as the number of millilitres (mL) of pure ethanol present in of solution at . The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at , which is . The ABV standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures. In some countries, e.g. France, alcohol by volume is often referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac), although there is a slight difference since the Gay-Lussac convention uses the International Standard Atmosphere value for temperature, . Volume change Mixing two solutions of alcohol of different strengths usually causes a change in volume. Mixing pure water w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt%''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the total mass of a samp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |