|
Ureidopenicillin
The ureidopenicillins are a group of penicillins which are active against ''Pseudomonas aeruginosa''. There are three ureidopenicillins in clinical use: *Azlocillin *Piperacillin *Mezlocillin They are mostly ampicillin derivatives in which the amino acid side chain has been converted to a variety of cyclic ureas. It is speculated that the added side chain mimics a longer segment of the peptidoglycan chain, more than ampicillin, and thus would bind more easily to the penicillin-binding proteins. Ureidopenicillins are not resistant to beta-lactamases. They are used parenterally, and are particularly indicated in infections caused by Gram-negative bacteria Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am .... References {{Cell wall disruptive antibiotics Penicillins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperacillin
Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen ''Pseudomonas aeruginosa''. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin". When used alone, piperacillin lacks strong activity against the Gram-positive pathogens such as '' Staphylococcus aureus'', as the beta-lactam ring is hydrolyzed by the bacteria's beta-lactamase. It was patented in 1974 and approved for medical use in 1981. Piperacillin is most commonly used in combination with the beta-lactamase inhibitor tazobactam ( piperacillin/tazobactam), which enhances piperacillin's effectiveness by inhibiting many beta lactamases to which it is susceptible. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from '' Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G (intramuscular or intravenous use) and penicillin V (given by mouth). Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use. 10% of the population claims penicillin allergies but because the frequency of positive skin test results decreases by 10% with each year of avoidance, 90% of these patients can tolerate penicillin. Additionally, those wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
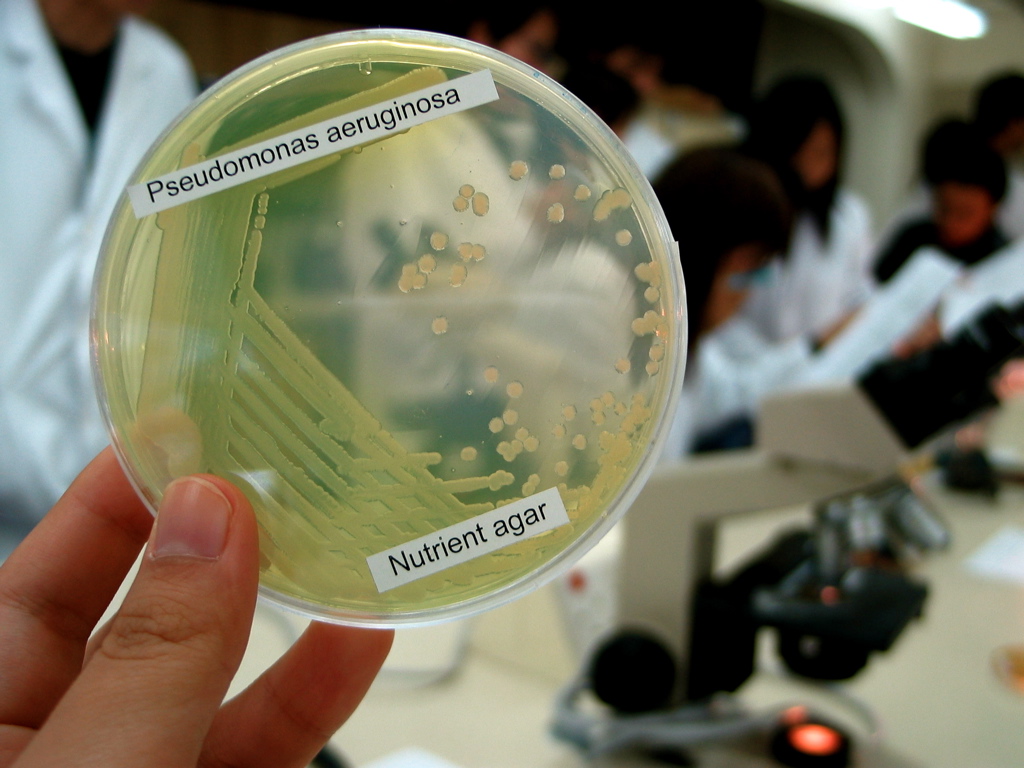
Pseudomonas Aeruginosa
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aeruginosa'' is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. The organism is considered opportunistic insofar as serious infection often occurs during existing diseases or conditions – most notably cystic fibrosis and traumatic burns. It generally affects the immunocompromised but can also infect the immunocompetent as in hot tub folliculitis. Treatment of ''P. aeruginosa'' infections can be difficult due to its natural resistance to antibiotics. When more advanced antibiotic drug regimens are needed adverse effects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism ''Escherichia coli'', as well as many pathogenic bacteria, such as ''Pseudomonas aeruginosa'', '' Chlamydia trachomatis'', and '' Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azlocillin Skeletal
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater ''in vitro'' potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including ''Pseudomonas aeruginosa'' and, in contrast to most cephalosporins, exhibits activity against enterococci. Spectrum of bacterial susceptibility Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms. * ''Escherichia coli'' 1 μg/mL – 32 μg/mL * ''Haemophilus'' spp. 0.03 μg/mL – 2 μg/mL * ''Pseudomonas aeruginosa'' 4 μg/mL – 6.25 μg/mL Synthesis An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chloride (2) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mezlocillin
Mezlocillin is a broad-spectrum penicillin antibiotic. It is active against both Gram-negative and some Gram-positive bacteria. Unlike most other extended spectrum penicillins, it is excreted by the liver, therefore it is useful for biliary tract infections, such as ascending cholangitis. Mechanism of action Like all other beta-lactam antibiotics, mezlocillin inhibits the third and last stage of bacterial cell wall synthesis by binding to penicillin binding proteins. This ultimately leads to cell lysis. Susceptible organisms Gram-negative *''Bacteroides'' spp., including ''B. fragilis'' *''Enterobacter'' spp. *''Escherichia coli'' *''Haemophilus influenzae'' *''Klebsiella'' species *'' Morganella morganii'' *'' Neisseria gonorrhoeae'' *''Proteus mirabilis'' *''Proteus vulgaris'' *'' Providencia rettgeri'' *''Pseudomonas'' spp., including '' P. aeruginosa'' *''Serratia marcescens'' Gram-positive *''Enterococcus faecalis'' *'' Peptococcus'' spp.'' *''Peptostreptococcus'' spp. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azlocillin
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater ''in vitro'' potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including '' Pseudomonas aeruginosa'' and, in contrast to most cephalosporins, exhibits activity against enterococci. Spectrum of bacterial susceptibility Azlocillin is considered a broad spectrum antibiotic and can be used against a number of Gram positive and Gram negative bacteria. The following represents MIC susceptibility data for a few medically significant organisms. * ''Escherichia coli'' 1 μg/mL – 32 μg/mL * ''Haemophilus'' spp. 0.03 μg/mL – 2 μg/mL * ''Pseudomonas aeruginosa'' 4 μg/mL – 6.25 μg/mL Synthesis An interesting alternative synthesis of azlocillin involves activation of the substituted phenylglycine analogue 1 with 1,3-dimethyl-2-chloro-1-imidazolinium chlorid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ampicillin
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously. Common side effects include rash, nausea, and diarrhea. It should not be used in people who are allergic to penicillin. Serious side effects may include ''Clostridium difficile'' colitis or anaphylaxis. While usable in those with kidney problems, the dose may need to be decreased. Its use during pregnancy and breastfeeding appears to be generally safe. Ampicillin was discovered in 1958 and came into commercial use in 1961. It is on the World Health Organization's List of Essential Medicines. The World Health Organization classifies ampicillin as critically important for human medicine. It is available as a generic medication. Medical u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important raw material for the chemical industry. In 1828 Friedrich Wöhler discovered that urea can be produced from inorganic starting materials, which was an important conceptual miles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidoglycan
Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer outside the plasma membrane, the rigid cell wall (murein sacculus) characteristic of most bacteria (domain ''Bacteria''). The sugar component consists of alternating residues of β-(1,4) linked ''N''-acetylglucosamine (NAG) and ''N''-acetylmuramic acid (NAM). Attached to the ''N''-acetylmuramic acid is a oligopeptide chain made of three to five amino acids. The peptide chain can be cross-linked to the peptide chain of another strand forming the 3D mesh-like layer. Peptidoglycan serves a structural role in the bacterial cell wall, giving structural strength, as well as counteracting the osmotic pressure of the cytoplasm. This repetitive linking results in a dense peptidoglycan layer which is critical for maintaining cell form and withstanding high osmotic pressures, and it is regularly replaced by peptidoglycan production. Peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin-binding Proteins
Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics (except for tabtoxinine-β-lactam, which inhibits glutamine synthetase) bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases. Diversity There are a large number of PBPs, usually several in each organism, and they are found as both membrane-bound and cytoplasmic proteins. For example, Spratt (1977) reports that six different PBPs are routinely detected in all strains of '' E. coli'' ranging in molecular weight from 40,000 to 91,000. The different PBPs occur in different numbers per cell and have varied affinities for penicillin. The PBPs are usually broadly classified into high-molecula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |