|
Unbiunium
Unbiunium, also known as eka-actinium or element 121, is a hypothetical chemical element; it has symbol Ubu and atomic number 121. ''Unbiunium'' and ''Ubu'' are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the first of the superactinides, and the third element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability. It is also likely to be the first of a new g-block of elements. Unbiunium has not yet been synthesized. It is expected to be one of the last few reachable elements with current technology; the limit could be anywhere between element 120 and 124. It will also likely be far more difficult to synthesize than the elements known so far up to 118, and still more difficult than elements 119 and 120. The teams at RIKEN in Japan and at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superactinide
An extended periodic table theorizes about chemical elements beyond those currently known and proven. The element with the highest atomic number known is oganesson (''Z'' = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical. Elements beyond 118 will be placed in additional periods when discovered, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements. Any additional periods are expected to contain more elements than the seventh period, as they are calculated to have an additional so-called ''g-block'', containing at least 18 elements with partially filled g- orbitals in each period. An ''eight-period table'' containing this block was suggested by Glenn T. Seaborg in 1969. The first element of the g-block may have atomic number 121, and thus would have the systematic name unbiunium. Despite many searches, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheavy Element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 element, group 3 adopted by authors, lawrencium may also be included to complete the 6d series. Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to unbiunium, 121 and a superactinide series approximately spanning elements unbibium, 122 to 153 (though more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ununennium
Ununennium, also known as eka-francium or element 119, is a hypothetical chemical element; it has symbol Uue and atomic number 119. ''Ununennium'' and ''Uue'' are the temporary systematic element name, systematic IUPAC name and symbol respectively, which are used until the element has been discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkali metal, and the first element in the eighth periodic table period, period. It is the lightest element that has not yet been synthesized. An attempt to synthesize the element has been ongoing since 2018 in RIKEN in Japan. The Joint Institute for Nuclear Research in Dubna, Russia, plans to make an attempt at some point in the future, but a precise date has not been released to the public. The Heavy Ion Research Facility in Lanzhou, China (HIRFL) also plans to make an attempt. Theoretical and experimental evidence has shown that the synthesis of unune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematic Element Name
A systematic element name is the temporary name assigned to an unknown or recently synthesized chemical element. A systematic symbol is also derived from this name. In chemistry, a transuranic element receives a permanent name and symbol only after its synthesis has been confirmed. In some cases, such as the Transfermium Wars, controversies over the formal name and symbol have been protracted and highly political. In order to discuss such elements without ambiguity, the International Union of Pure and Applied Chemistry (IUPAC) uses a set of rules, adopted in 1978, to assign a temporary systematic name and symbol to each such element. This approach to naming originated in the successful development of regular rules for the naming of organic compounds. IUPAC rules The temporary names derive systematically from the element's atomic number, and apply only to 101 ≤ ''Z'' ≤ 999. Each digit is translated into a "numerical root" according to the table. The root ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
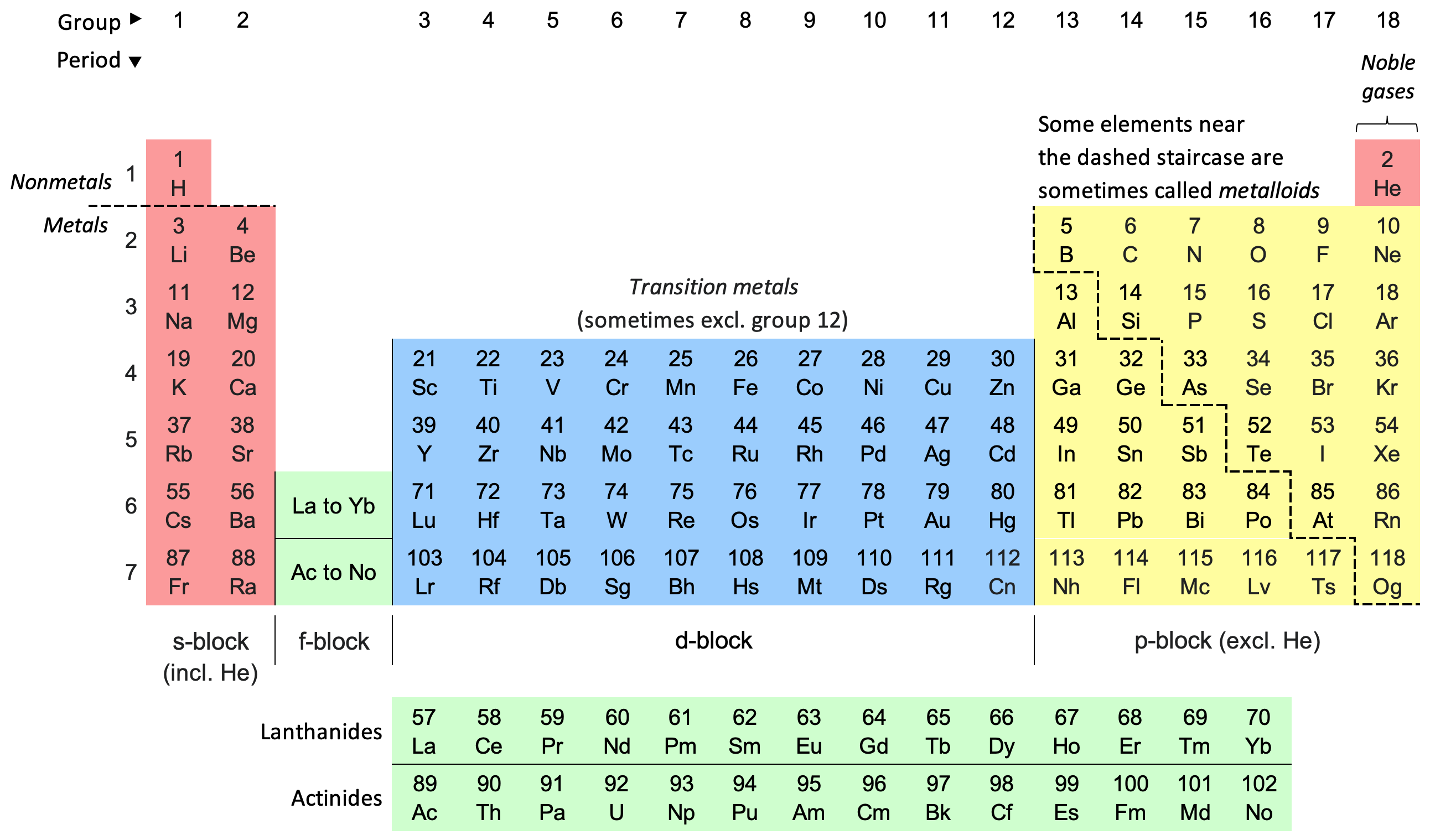
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Block (periodic Table)
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term seems to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal quantum number: sharp (0), principal (1), diffuse (2), and fundamental (3). Succeeding notations proceed in alphabetical order, as g, h, etc., though elements that would belong in such blocks have not yet been found. Characteristics There is an ''approximate'' correspondence between this nomenclature of blocks, based on electronic configuration, and sets of elements based on chemical properties. The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block corresponds to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unbiquadium
Unbiquadium, also known as element 124 or eka-uranium, is a hypothetical chemical element; it has placeholder symbol Ubq and atomic number 124. ''Unbiquadium'' and ''Ubq'' are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbiquadium is expected to be a g-block superactinide and the sixth element in the 8th period. Unbiquadium has attracted attention, as it may lie within the island of stability, leading to longer half-lives, especially for 308Ubq which is predicted to have a magic number of neutrons (184). Despite several searches, unbiquadium has not been synthesized, nor have any naturally occurring isotopes been found to exist. It is believed that the synthesis of unbiquadium will be far more challenging than that of lighter undiscovered elements, and nuclear instability may pose further difficulties in identifying unbiquadium, unless the island of stability has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the release or absorption (electromagnetic radiation), absorption of energy. This difference in mass arises as a result of the difference in nuclear binding energy between the atomic nuclei before and after the fusion reaction. Nuclear fusion is the process that powers all active stars, via many Stellar nucleosynthesis, reaction pathways. Fusion processes require an extremely large Lawson criterion, triple product of temperature, density, and confinement time. These conditions occur only in Stellar core, stellar cores, advanced Nuclear weapon design, nuclear weapons, and are approached in List of fusion experiments, fusion power experiments. A nuclear fusion process that produces atomic nuclei lighter than nickel-62 is generally exothermic, due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cold Fusion
Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature. It would contrast starkly with the nuclear fusion, "hot" fusion that is known to take place naturally within Main sequence, stars and artificially in Thermonuclear weapon, hydrogen bombs and prototype Fusion power, fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion. There is currently no accepted theoretical model that would allow cold fusion to occur. In 1989, two electrochemistry, electrochemists at the University of Utah, Martin Fleischmann and Stanley Pons, reported that their apparatus had produced anomalous heat ("excess heat") of a magnitude they asserted would defy explanation except in terms of nuclear processes. They further reported measuring small amounts of nuclear reaction byproducts, including neutrons and tritium. ("It is inconceivable that this [amount of heat] could be due to anyth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part of the 6d transition series. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. The 1985 IUPAC nomenclature of inorganic chemistry, IUPAC ''Red Book'' recommends that ''actinoid'' be used rather than ''actinide'', since the suffix ''-ide'' normally indicates a negative ion. However, owing to widespread current use, ''actinide'' is still allowed. Actinium through nobelium are f-block elements, while lawrencium is a d-block element and a transition metal. The series mostly corresponds to the filling of the 5f electron shell, although as isolated atoms in the ground state many have anomalous configu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Period 4 Element
A period 4 element is one of the chemical elements in the fourth row (or Period (periodic table), period) of the periodic table, periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fourth period contains 18 elements #s-block elements, beginning with potassium and ending with #Krypton, krypton – one element for each of the group (periodic table), eighteen groups. It sees the first appearance of d-block (which includes transition metals) in the table. Properties All 4th-period elements are Radioactive element, stable, and many are extremely common in the Earth's crust and/or Earth's core, core; it is the last period with no unstable elements. Many transition metals in the period are very ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |