|
Thermal Physics
Thermal physics is the combined study of thermodynamics, statistical mechanics, and kinetic theory of gases. This umbrella-subject is typically designed for physics students and functions to provide a general introduction to each of three core heat-related subjects. Other authors, however, define thermal physics loosely as a summation of only thermodynamics and statistical mechanics. Thermal physics can be seen as the study of system with larger number of atom, it unites thermodynamics to statistical mechanics. Overview Thermal physics, generally speaking, is the study of the statistical nature of physical systems from an energetic perspective. Starting with the basics of heat and temperature, thermal physics analyzes the first law of thermodynamics and second law of thermodynamics from the statistical perspective, in terms of the number of microstates corresponding to a given macrostate. In addition, the concept of entropy is studied via quantum theory. A central topic in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
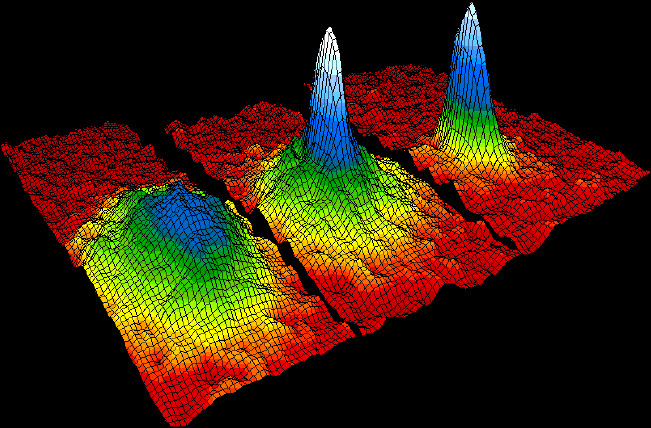
Bose Einstein Condensate
{{disambiguation, geo, given name ...
Bose may refer to: * Bose (crater), a lunar crater * ''Bose'' (film), a 2004 Indian Tamil film starring Srikanth and Sneha * Bose (surname), a surname (and list of people with the name) * Bose, Italy, a ''frazioni'' in Magnano, Province of Biella ** Bose Monastic Community, a monastic community in the village * Bose, Poland * Bose Corporation, an audio company * Bose Ogulu, Nigerian manager of Burna Boy * Baise, or Bose, a prefecture-level city in Guangxi, China See also * * Boise (other) * Bos (other) * Bose–Einstein (other), relating to Satyendra Nath Bose * Basu Basu (variants: '' Bose, Boshu, Bosu, Bosh'') is an Indian surname, primarily found among Bengali Hindus. It stems from Sanskrit वासु vāsu (a name of Vishṇu meaning 'dwelling in all beings'). History Basus belong to the Kayastha c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermion
In particle physics, a fermion is a particle that follows Fermi–Dirac statistics. Generally, it has a half-odd-integer spin: spin , spin , etc. In addition, these particles obey the Pauli exclusion principle. Fermions include all quarks and leptons and all composite particles made of an odd number of these, such as all baryons and many atoms and nuclei. Fermions differ from bosons, which obey Bose–Einstein statistics. Some fermions are elementary particles (such as electrons), and some are composite particles (such as protons). For example, according to the spin-statistics theorem in relativistic quantum field theory, particles with integer spin are bosons. In contrast, particles with half-integer spin are fermions. In addition to the spin characteristic, fermions have another specific property: they possess conserved baryon or lepton quantum numbers. Therefore, what is usually referred to as the spin-statistics relation is, in fact, a spin statistics-quantum nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Information Theory
Information theory is the scientific study of the quantification, storage, and communication of information. The field was originally established by the works of Harry Nyquist and Ralph Hartley, in the 1920s, and Claude Shannon in the 1940s. The field is at the intersection of probability theory, statistics, computer science, statistical mechanics, information engineering, and electrical engineering. A key measure in information theory is entropy. Entropy quantifies the amount of uncertainty involved in the value of a random variable or the outcome of a random process. For example, identifying the outcome of a fair coin flip (with two equally likely outcomes) provides less information (lower entropy) than specifying the outcome from a roll of a die (with six equally likely outcomes). Some other important measures in information theory are mutual information, channel capacity, error exponents, and relative entropy. Important sub-fields of information theory include sourc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer Physics
Heat transfer physics describes the kinetics of energy storage, transport, and energy transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is different made (converted) among various carriers. The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Conduction
Conduction is the process by which heat is transferred from the hotter end to the colder end of an object. The ability of the object to conduct heat is known as its ''thermal conductivity'', and is denoted . Heat spontaneously flows along a temperature gradient (i.e. from a hotter body to a colder body). For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becoming more uniform. In conduction, the heat flow is within and through the body itself. In contrast, in heat transfer by thermal radiation, the transfer is often between bodies, which may be separated spatially. Heat can also be transferred by a combination of conduction and radiation. In solids, conduction is mediated by the combination of vibrations and collisions of molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mean Free Path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a result of one or more successive collisions with other particles. Scattering theory Imagine a beam of particles being shot through a target, and consider an infinitesimally thin slab of the target (see the figure). The atoms (or particles) that might stop a beam particle are shown in red. The magnitude of the mean free path depends on the characteristics of the system. Assuming that all the target particles are at rest but only the beam particle is moving, that gives an expression for the mean free path: :\ell = (\sigma n)^, where is the mean free path, is the number of target particles per unit volume, and is the effective cross-sectional area for collision. The area of the slab is , and its volume is . The typical number of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the Celsius scale ( International System of Units), Note: The triple point of water is 0.01 °C, not 0 °C; thus 0 K is −2890.15 °C, not −273.16 °C. which equals −459.67 degrees on the Fahrenheit scale (United States customary units or Imperial units). The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition. It is commonly thought of as the lowest temperature possible, but it is not the lowest ''enthalpy'' state ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equipartition Theorem
In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in translational motion of a molecule should equal that in rotational motion. The equipartition theorem makes quantitative predictions. Like the virial theorem, it gives the total average kinetic and potential energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values of individual components of the energy, such as the kinetic energy of a particular particle or the potential energy of a single spring. For example, it predicts that every atom in a monatomic ideal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Equilibrium
In thermodynamics, the phase rule is a general principle governing "pVT" systems, whose thermodynamic states are completely described by the variables pressure (), volume () and temperature (), in thermodynamic equilibrium. If is the number of degrees of freedom, is the number of components and is the number of phases, then :F = C - P + 2 It was derived by American physicist Josiah Willard Gibbs in his landmark paper titled '' On the Equilibrium of Heterogeneous Substances'', published in parts between 1875 and 1878. The rule assumes the components do not react with each other. The number of degrees of freedom is the number of independent intensive variables, i.e. the largest number of thermodynamic parameters such as temperature or pressure that can be varied simultaneously and arbitrarily without determining one another. An example of one-component system is a system involving one pure chemical, while two-component systems, such as mixtures of water and ethanol, have tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, rates of the forward and backward (reverse) reactions must be equal. In the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |