|
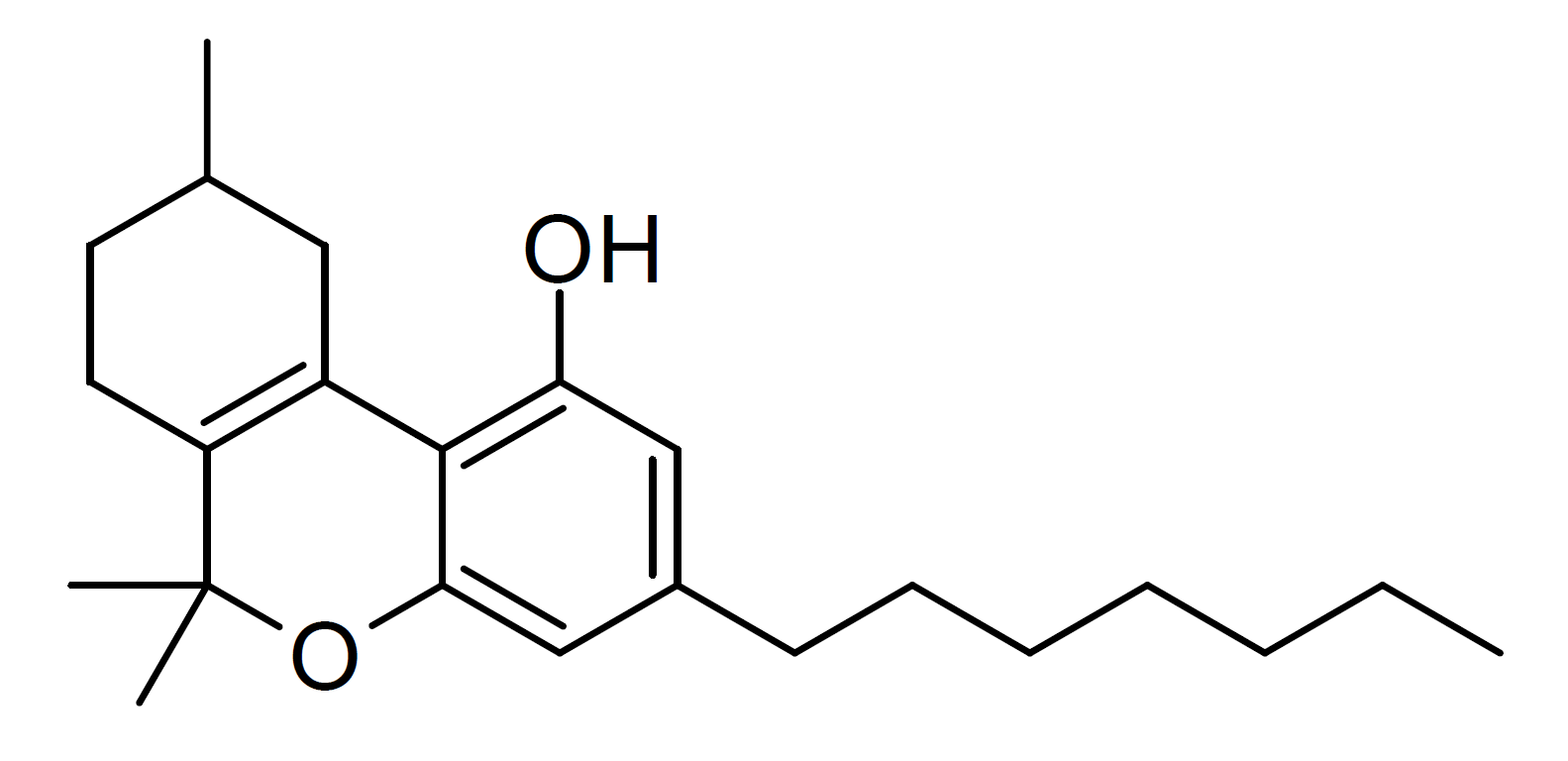
Tetrahydrocannabiphorol
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis sativa''. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity approximately 33 times that of Delta-9-THC. Isomers Delta-3-THCP ] The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Delta-3-Tetrahydrocannabinol, Δ3-THC, unlike the hexyl homologue parahexyl which was significantly stronger. Delta-8-THCP The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091, It's unconfirmed whether or not Delta-8-THCP is found naturally in cannabis plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CBD-DMH
Cannabidiol-dimethylheptyl (CBD-DMH or DMH-CBD) is a synthetic homologue of cannabidiol where the pentyl chain has been replaced by a dimethylheptyl chain. Several isomers of this compound are known. The most commonly used isomer in research is (−)-CBD-DMH, which has the same stereochemistry as natural cannabidiol, and a 1,1-dimethylheptyl side chain. This compound is not psychoactive and acts primarily as an anandamide reuptake inhibitor, but is more potent than cannabidiol as an anticonvulsant and has around the same potency as an antiinflammatory. Unexpectedly the “unnatural” enantiomer (+)-CBD-DMH, which has reversed stereochemistry from cannabidiol, was found to be a directly acting cannabinoid receptor agonist with a Ki of 17.4nM at CB1 and 211nM at CB2, and produces typical cannabinoid effects in animal studies, as does its 7-OH derivative. Another closely analogous compound has also been described, with the double bond in the cyclohexene ring shifted to between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delta-8-THC
Delta-8-tetrahydrocannabinol (delta-8-THC, Δ8-THC) is a psychoactive cannabinoid found in the Cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol (delta-9-THC, Δ9-THC), the compound commonly known as THC. ∆8-THC is under preliminary research for its biological properties. Effects ∆8-THC is moderately less potent than Δ9-THC. This essentially means that it has properties similar to those of ∆9-THC, although to a lesser degree per milligram of material consumed. Delta-8-THC and delta-9-THC both contain a double bond in their molecular structure, but the location is different. Delta-8-THC has the bond in the eighth carbon while delta-9 contains it in the 9th carbon. Although ∆8-THC functions similarly to Δ9-THC in many ways, it appears to be only two-thirds as psychoactive. This may be because it binds differently to CB1, the cannabinoid receptor that regulates much of THC’s mind-altering effect. ∆8-THC may cause increased heart rate, reddening of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DMHP
Dimethylheptylpyran (DMHP, 3-(1,2-dimethylheptyl)-Δ6a(10a)-THC, 1,2-dimethylheptyl-Δ3-THC, A-40824, or EA-2233) is a synthetic analog of THC, which was invented in 1949 during attempts to elucidate the structure of Δ9-THC, one of the active components of ''Cannabis''. DMHP is a pale yellow, viscous oil which is insoluble in water but dissolves in alcohol or non-polar solvents. Effects DMHP is similar in structure to THC, differing only in the position of one double bond, and the replacement of the 3-pentyl chain with a 3-(1,2-dimethylheptyl) chain. It produces similar activity to THC, such as sedative effects, but is considerably more potent, especially having much stronger analgesic and anticonvulsant effects than THC, although comparatively weaker psychological effects. It is thought to act as a CB1 agonist, in a similar manner to other cannabinoid derivatives. While DMHP itself has been subject to relatively little study since the characterization of the canna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabihexol
Tetrahydrocannabihexol (Δ9-THCH, Δ9-Parahexyl, n-Hexyl-Δ9-THC) is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from ''Cannabis'' plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in ''Cannabis'', but likely is due to Delta-8-THC itself being a degraded form of Delta-9-THC. ] ] See also * Cannabidiphorol * Tetrahydrocannabiorcol * Tetrahydrocannabivarin * Tetrahydrocannabutol * Tetrahydrocannabiphorol Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabutol
Δ9-Tetrahydrocannabutol (tetrahydrocannabinol-C4, THC-C4, Δ9-THCB, (C4)-Δ9-THC, butyl-THC) is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. Pharmacology Δ9-THCB, showed an affinity for the human CB1 (''K''i = 15 nM) and CB2 receptors (''K''i = 51 nM) comparable to that of Δ9-THC. The formalin test in vivo was performed on Δ9-THCB in order to reveal possible analgesic and anti-inflammatory properties. The tetrad test in mice showed a partial agonistic activity of Δ9-THCB toward the CB1 receptor. THCB has rarely been isolated from cannabis samples, but appears to be less commonly present than THC or THCV. It is metabolized in a similar manner to THC. In an analysis by the University of Rhode Island on phytocannabinoids it was found that THC-Butyl had the highest 3C-like protease inhibitor a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabivarin
Tetrahydrocannabivarin (THCV, THV, O-4394, GWP42004) is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of a pentyl (5-carbon) group on the molecule, which makes it produce very different effects from THC. Natural occurrence THCV is prevalent in certain central Asian and southern African strains of ''Cannabis''. Chemistry Similar to THC, THCV has 7 possible double bond isomers and 30 stereoisomers (see: Tetrahydrocannabinol#Isomerism). The alternative isomer Δ8-THCV is known as a synthetic compound with a code number of O-4395, but it is not known to have been isolated from ''Cannabis'' plant material. ] Description Plants with elevated levels of propyl cannabinoids (including THCV) have been found in populations of ''Cannabis sativa'' L. ssp. ''indica'' (= ''Cannabis indica'' Lam.) from China, India, Nepal, Thailand, Afghanistan, and Pakistan, as well as southern and western Africa. THCV levels up to 53.7% of total cannabinoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parahexyl
Parahexyl (Synhexyl, n-hexyl-Δ3-THC, (C6)-Δ6a(10a)-THC) is a synthetic homologue of THC which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. Parahexyl is similar in both structure and activity to THC, differing only in the position of one double bond and the lengthening of the chain by one CH2 group to . Parahexyl produces effects typical of other cannabinoid receptor agonists in animals. It has a somewhat higher oral bioavailability than THC itself but is otherwise very similar. Presumably, it acts as a CB1 agonist in the same way as THC, but as there has been no research published using parahexyl since the discovery of the CB1 receptor, this has not been definitively confirmed. Parahexyl was occasionally used as an anxiolytic in the mid-20th century, the dosage ranging from 5 mg to 90 mg. Parahexyl was made illegal under UN convention in 1982 on the basis of its structural similarity and sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parahexyl
Parahexyl (Synhexyl, n-hexyl-Δ3-THC, (C6)-Δ6a(10a)-THC) is a synthetic homologue of THC which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. Parahexyl is similar in both structure and activity to THC, differing only in the position of one double bond and the lengthening of the chain by one CH2 group to . Parahexyl produces effects typical of other cannabinoid receptor agonists in animals. It has a somewhat higher oral bioavailability than THC itself but is otherwise very similar. Presumably, it acts as a CB1 agonist in the same way as THC, but as there has been no research published using parahexyl since the discovery of the CB1 receptor, this has not been definitively confirmed. Parahexyl was occasionally used as an anxiolytic in the mid-20th century, the dosage ranging from 5 mg to 90 mg. Parahexyl was made illegal under UN convention in 1982 on the basis of its structural similarity and sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexahydrocannabinol
Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol. It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in ''Cannabis sativa'', but can also be produced synthetically by hydrogenation of cannabis extracts. HHC was first synthesized in 1947 by Roger Adams using natural THC found in ''Cannabis sativa''. Several research groups have successfully synthesized (+)-HHC and (-)-HHC using citronellal and olivetol, as well as other related compounds. While similar compounds have previously been identified in cannabis, hexahydrocannabinol itself has rarely been isolated from the plant. The de Las Heras group in 2020 took lipid extract from ''Cannabis sativa'' seeds and discovered 43 cannabinoids in the crude extract; one of them being hexahydrocannabinol. It has two diastereomers at the methyl (9) position. HHC is typically made from Δ8-THC, or Δ9-THC. There are no double bonds in the cyclohexyl ring like D8/D9 hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CB1 Receptor Agonists
{{Letter-NumberCombDisambig ...
CB1 may refer to: * CB1, a postcode district in the CB postcode area * Cannabinoid receptor 1, a receptor for cannabinoids in the brain * ''Crash Bandicoot'' (video game), the first game in the ''Crash Bandicoot'' series * Manhattan Community Board 1 The Manhattan Community Board 1 is a New York City community board encompassing the neighborhoods of Battery Park City, the Financial District, the South Street Seaport, and TriBeCa in Lower Manhattan in the borough of Manhattan as well as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabicyclohexanol
Cannabicyclohexanol (CCH, CP 47,497 dimethyloctyl homologue, (C8)-CP 47,497) is a cannabinoid receptor agonist drug, developed by Pfizer in 1979. On 19 January 2009, the University of Freiburg in Germany announced that an analog of CP 47,497 was the main active ingredient in the herbal incense product ''Spice'', specifically the 1,1-dimethyloctyl homologue of CP 47,497, which is now known as cannabicyclohexanol. The 1,1-dimethyloctyl homologue of CP 47,497 is in fact several times more potent than the parent compound, which is somewhat unexpected as the 1,1-dimethylheptyl is the most potent substituent in classical cannabinoid compounds such as HU-210. Enantiomers Cannabicyclohexanol has four enantiomers, which by analogy with other related cannabinoid compounds can be expected to have widely varying affinity for cannabinoid receptors, and consequently will show considerable variation in potency. While the (-)-''cis'' enantiomer (-)-cannabicyclohexanol discovered in the original ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1871
O-1871 is a potent cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It has a CB1 receptor affinity of 2.0nM and a CB2 receptor affinity of 0.3nM. Structurally, O-1871 is a cyclohexylphenol derivative related to CP 47,497, and so is illegal in some jurisdictions where CP 47,497 and its derivatives are banned. However the 3,3-dimethylcyclohexyl substituent of O-1871 can be replaced by various other groups, producing other potent compounds such as the cycloheptyl derivative O-1656 and the 2-adamantyl derivative O-1660, as well as the corresponding 3,5-dichlorophenyl derivative, which are not cyclohexylphenol derivatives. ] ] See also * CP 55,940 * Cannabidiol Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. , clinical research on CBD in ... * Cannabicyclohexanol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |