|
TRIM21
Tripartite motif-containing protein 21, also known as E3 ubiquitin-protein ligase TRIM21, is a protein that in humans is encoded by the ''TRIM21'' gene. Alternatively spliced transcript variants for this gene have been described but the full-length nature of only one has been determined. It is expressed in most human tissues. Structure TRIM21 is a member of the tripartite motif family, tripartite motif (TRIM) family. The TRIM motif includes three zinc-binding domains, a RING finger domain, a B-box type 1 and a B-box type 2 zinc finger, and a coiled coil region. Function TRIM21 is an intracellular antibody effector in the intracellular antibody-mediated proteolysis pathway. It recognizes Fc-domain, Fc domain and binds to immunoglobulin G, immunoglobulin A and immunoglobulin M on antibody marked non-enveloped virions which have infected the cell. Either by Ubiquitin, autoubiquitination or by ubiquitination of a cofactor, it is then responsible for directing the virions to the protea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracellular Antibody-mediated Proteolysis
Intracellular antibody-mediated degradation (IAMD) is a neutralization mechanism of intracellular antibody-mediated immunity whereby an effector protein, TRIM21, directs antibody bound virus, virions to the proteasome where they are degraded. As yet, it has only been observed to act against the adenovirus but is likely to also be effective against other non-enveloped viruses. Mechanism of action In IAMD, the neutralization of the pathogen follows a non-cytotoxic mechanism. That is, the infected cell is not attacked as in Antibody-dependent cell-mediated cytotoxicity, instead the virions are rapidly destroyed and the cell may be relieved of infection. #Immunoglobulin G (IgG) binds specifically to the target antigen presented on the pathogen extracellularly #The antibody bound pathogen infects a host cell #In the cytosol, TRIM21 (a protein of the Tripartite motif family) binds with high affinity to IgG #TRIM21 is conjugated with ubiquitin, which directs the complex to the proteasome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
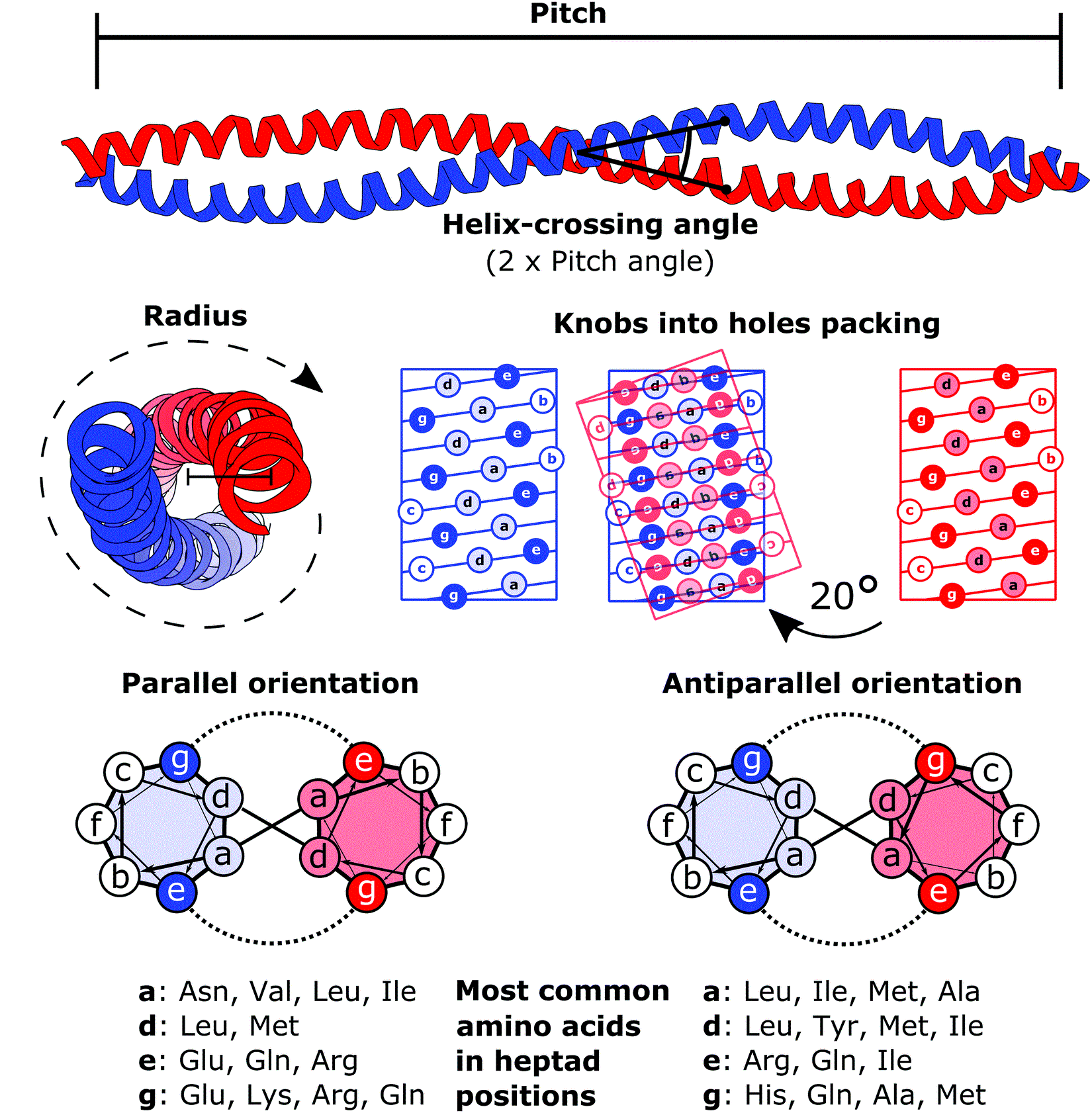
Tripartite Motif Family
The tripartite motif family (TRIM) is a protein family. Function Many TRIM proteins are induced by interferons, which are important component of resistance to pathogens and several TRIM proteins are known to be required for the restriction of infection by lentiviruses. TRIM proteins are involved in pathogen-recognition and by regulation of transcriptional pathways in host defence. Structure The tripartite motif is always present at the N-terminus of the TRIM proteins. The TRIM motif includes the following three domains: * (1) a RING finger domain * (2) one or two B-box zinc finger domains ** when only one B-box is present, it is always a type-2 B-box ** when two B-boxes are present the type-1 B-Box always precedes the type-2 B-Box * (3) coiled coil region The C-terminus of TRIM proteins contain either: * Group 1 proteins: a C-terminal domain selected from the following list: ** NHL and IGFLMN domains, either in association or alone ** PHD domain The PHD finger was discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin G
Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes. Function Antibodies are major components of humoral immunity. IgG is the main type of antibody found in blood and extracellular fluid, allowing it to control infection of body tissues. By binding many kinds of pathogens such as viruses, bacteria, and fungi, IgG protects the body from infection. It does this through several mechanisms: * IgG-mediated binding of pathogens causes their immobilization and binding together via agglutination; IgG coating of pathogen surfaces (known as opsonization) allows their recognition and ingestion by phagocytic immune cells leading to the elimination of the pathogen itself; * IgG activates the classical pathway of the complement system, a cascade of immune protein product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteasome
Proteasomes are essential protein complexes responsible for the degradation of proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are found inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, proteasomes are located both in the nucleus and in the cytoplasm. The proteasomal degradation pathway is essential for many cellular processes, including the cell cycle, the regulation of gene expression, and responses to oxidative stress. The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2004 Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko and Irwin Rose. The core 20S proteasome (blue in the adjacent figure) is a cylindrical, compartmental protein complex of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RING Finger Domain
In molecular biology, a RING (short for Really Interesting New Gene) finger domain is a protein structural domain of zinc finger type which contains a C3HC4 amino acid motif which binds two zinc cations (seven cysteines and one histidine arranged non-consecutively). This protein domain contains 40 to 60 amino acids. Many proteins containing a RING finger play a key role in the ubiquitination pathway. Conversely, proteins with RING finger domains are the largest type of ubiquitin ligases in the human genome. Zinc fingers Zinc finger (Znf) domains are relatively small protein motifs that bind one or more zinc atoms, and which usually contain multiple finger-like protrusions that make tandem contacts with their target molecule. They bind DNA, RNA, protein and/or lipid substrates. Their binding properties depend on the amino acid sequence of the finger domains and of the linker between fingers, as well as on the higher-order structures and the number of fingers. Znf domains a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Finger
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. The term ''zinc finger'' was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (''Xenopus laevis'') transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. '' Xenopus laevis'' TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in ''Drosophila''. It often appears as a metal-binding domain in multi-domain proteins. Proteins that contain zinc fingers (zinc finger proteins) are classified into several different structural families. Unlike many other clearly defined supersecondary structures such as Greek keys or β hairpins, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coiled Coil
A coiled coil is a structural motif in proteins in which two to seven alpha-helices are coiled together like the strands of a rope. ( Dimers and trimers are the most common types.) They have been found in roughly 5-10% of proteins and have a variety of functions. They are one of the most widespread motifs found in protein-protein interactions. To aid protein study, several tools have been developed to predict coiled-coils in protein structures. Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fc-domain
The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This region allows antibodies to activate the immune system, for example, through binding to Fc receptors. In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody's two heavy chains; IgM and IgE Fc regions contain three heavy chain constant domains (CH domains 2–4) in each polypeptide chain. The Fc regions of IgGs bear a highly conserved N-glycosylation site. Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity. The N-glycans attached to this site are predominantly core- fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues. The other part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin A
Immunoglobulin A (IgA, also referred to as sIgA in its secretory form) is an antibody that plays a role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body. IgA has two subclasses ( IgA1 and IgA2) and can be produced as a monomeric as well as a dimeric form. The IgA dimeric form is the most prevalent and, when it has bound the Secretory component, is also called ''secretory IgA'' (sIgA). sIgA is the main immunoglobulin found in mucous secretions, including tears, saliva, sweat, colostrum and secretions from the genitourinary tract, gastrointestinal tract, prostate and respiratory epithelium. It is also found in small amounts in blood. The secretory component of sIgA protects the immuno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin M
Immunoglobulin M (IgM) is the largest of several isotypes of antibodies (also known as immunoglobulin) that are produced by vertebrates. IgM is the first antibody to appear in the response to initial exposure to an antigen; causing it to also be called an acute phase antibody. In humans and other mammals that have been studied, plasmablasts in the spleen are the main source of specific IgM production. History In 1937, an antibody was observed in horses hyper-immunized with pneumococcus polysaccharide that was much larger in size than the typical rabbit γ-globulin, with a molecular weight of 990,000 daltons. In accordance with its larger size, the new antibody was originally referred to as γ-macroglobulin, and subsequently termed IgM—M for “macro”. The V domains of normal immunoglobulin are highly heterogeneous, reflecting their role in protecting against the great variety of infectious microbes, and this heterogeneity impeded detailed structural analysis of IgM. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |