|
TEMED
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, although old samples often appear yellow. Its odor is similar to that of rotting fish. As a reagent in synthesis TMEDA is widely employed as a ligand for metal ions. It forms stable complexes with many metal halides, e.g. zinc chloride and copper(I) iodide, giving complexes that are soluble in organic solvents. In such complexes, TMEDA serves as a bidentate ligand. TMEDA has an affinity for lithium ions. When mixed with ''n''-butyllithium, TMEDA's nitrogen atoms coordinate to the lithium, forming a cluster of higher reactivity than the tetramer or hexamer that ''n''-butyllithium normally adopts. BuLi/TMEDA is able to metallate or even doubly metallate many substrates including benzene, furan, thiophene, ''N''-alkylpyrroles, and ferrocen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
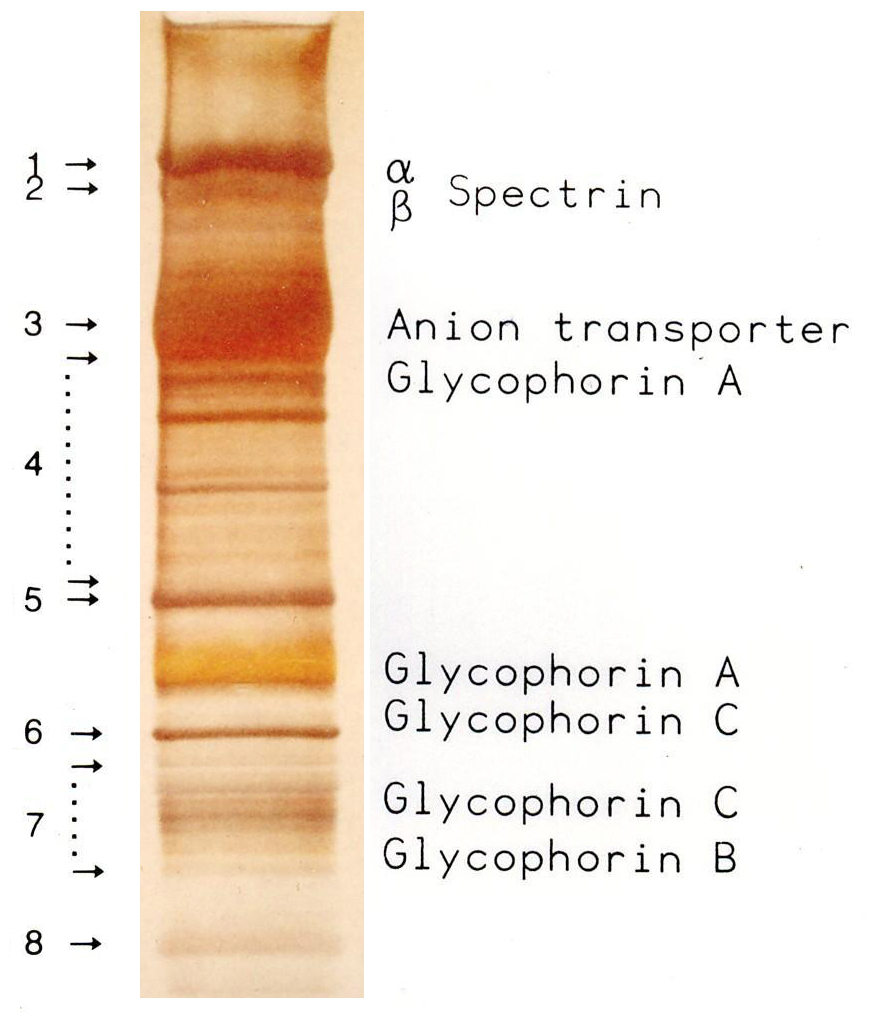
SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a Discontinuous electrophoresis, discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 Kilodalton, kDa. The combined use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall. Properties SDS-PAGE is an electrophoresis method that allows protein separation by mass. The medium (also referred to as ′matrix′) is a polyacrylamide-based discontinuous gel. The polyacrylamide-gel is typically sandwiched between two glass plates in a slab gel. Although tube gels (in glass cylinders) were used historically, they were rapid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylenetetramine
Triethylenetetramine (TETA and trien), also known as trientine ( INN) when used medically, is an organic compound with the formula H2NHCH2CH2NH2sub>2. The pure free base is a colorless oily liquid, but, like many amines, older samples assume a yellowish color due to impurities resulting from air oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA. The hydrochloride salts are used medically as a treatment for copper toxicity. Uses Epoxy uses The reactivity and uses of TETA are similar to those for the related polyamines ethylenediamine and diethylenetriamine. It is primarily used as a crosslinker ("hardener") in epoxy curing. TETA, like other aliphatic amines, react quicker and at lower temperatures than aromatic amines due to less negative steric effects since the linear nature of the molecule provides it the ability to rotate and twist. Medical uses The hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly Volatility (chemistry), volatile liquid with a boiling point close to room temperature. It is soluble in common organic Solvent, solvents, including ethanol, alcohol, diethyl ether, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamino Compounds
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one hydrogen. Dimethylamine is a base (chemistry), weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(dimethylamino)methane
Bis(dimethylamino)methane is the organic compound with the formula CH3)2Nsub>2CH2. It is classified as an aminal as well as a ditertiary amine, in fact the simplest. It is a colorless liquid that is widely available. It is prepared by the reaction of dimethylamine and formaldehyde Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...: : 2 (CH3)2NH + CH2O → CH3)2Nsub>2CH2 + H2O It is used for the dimethylaminomethylation reactions, the reaction being initiated by the addition of a strong, anhydrous acid: : CH3)2Nsub>2CH2 + H+ → (CH3)2NCH2+ + (CH3)2NH Bis(dimethylamino)methane, being a Lewis base, functions as a bidentate ligand.{{cite journal , doi=10.1002/chem.201600810 , title=Siloxymethylamines as Aminomethylation Reagents for Amines Leading to Labile Diamino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doyle Catalyst
Doyle is a surname of Irish origin. The name is a back-formation from O'Doyle, which is an Anglicisation of the Irish (), meaning "descendant of ''Dubhghall''". There is another possible etymology: the Anglo-Norman surname ''D'Oyley'' with agglutination of the French article ''de'' (cf. Disney). It means 'from Ouilly', the name of a knight who originated from one of the places named Ouilly in Normandy, such as Ouilly-le-Tesson (Calvados, ''Oylley'' 1050), Ouilly-le-Vicomte (Calvados, ''de Oilleio'' 1279), etc. The relationship with the family D'Oyly is unknown. The personal name ''Dubhghall'' contains the elements ''dubh'' "black" + ''gall'' "stranger". Similar Scottish and Irish surnames, derived from the same personal name are: '' MacDougall'' / '' McDougall'' and '' MacDowell'' / '' McDowell''. During the Viking Age the term '' Dubhghoill'' was used to describe the Vikings—usually Danes—and the term ''Fionnghoill'' ("fair foreigners") was used to describe Norwegians ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylethylenediamine(dimethyl)nickel(II)
Tetramethylethylenediamine(dimethyl)nickel(II) is the organonickel complex with the formula (Me = CH3). This yellow-brown, air-sensitive compound is popular precursor to diverse organonickel complexes. It is prepared from the tmeda adduct of nickel(II) acetylacetonate by reaction with methyl lithium.{{cite journal , doi=10.1016/0022-328X(88)89050-8, title=Tmeda-Nickel-Komplexe , year=1988 , last1=Kaschube , first1=Wilfried , last2=Pörschke , first2=Klaus R. , last3=Wilke , first3=Günther , journal=Journal of Organometallic Chemistry , volume=355 , issue=1–3 , pages=525–532 The tmeda ligand is easily displaced by bases such as bipyridine and diphosphines Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of .... Treatment of the complex with electrophilic alkenes results in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacrylamide
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries. Physicochemical properties Polyacrylamide is a polyolefin. It can be viewed as polyethylene with amide substituents on alternating carbons. Unlike various nylons, polyacrylamide is not a polyamide because the amide groups are not in the polymer backbone. Owing to the presence of the amide (CONH2) groups, alternating carbon atoms in the backbone are stereogenic (colloquially: chiral). For this reason, polyacrylamide exists in atactic, syndiotactic, and isotactic forms, although this aspect is rarely discussed. The polymerization is initiated with radicals and is assumed to be stereorandom. Copolymers and modified polymers Linear polyacrylamide is a water-soluble polymer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sec-Butyllithium
''sec''-Butyllithium is an organometallic compound with the Chemical formula, formula CH3CHLiCH2CH3, abbreviated ''sec''-BuLi or ''s''-BuLi. This Chiral (chemistry), chiral organolithium reagent is used as a source of ''sec''-butyl carbanion in organic synthesis.. Synthesis ''sec''-BuLi can be prepared by the reaction of ''sec''-butyl halides with lithium metal: Properties Physical properties ''sec''-Butyllithium is a colorless viscous liquid. Using mass spectrometry, it was determined that the pure compound has a tetrameric structure. It also exists as tetramers when dissolved in organic solvents such as benzene, cyclohexane or cyclopentane. The cyclopentane solution has been detected with 6Li-NMR spectroscopy to have a hexameric structure at temperatures below −41 °C. In electron-donating solvents such as tetrahydrofuran, there exists an equilibrium between monomeric and dimeric forms. Chemical properties The carbon-lithium bond is highly polar, rendering the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Salt
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2023 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 14.4. Editors-in-chief The following people are or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |